39 Pain and Nociception
Pain is a complex experience that has somatosensory, psychological, and affective components. Nociception is a sensory process that provides signals that trigger pain when in the presence of a noxious stimulus. Detection and avoidance of pain is a highly-adaptive behavior that can improve the odds of survival chances of an animal. When we feel pain, we are more likely to protect the injured site to prevent further damage, making the sensation of pain beneficial to our overall well-being.
Nociceptors
Pain detection is carried out by nociceptors that are located in the periphery at the surface of the skin. Nociceptors can detect a variety of noxious stimuli, ranging from crush (mechanical), acid (chemical), and high heat (thermal). They are expressed on free nerve endings that innervate the skin. Many pain-detecting neurons respond to more than one type of noxious stimulus and are called polymodal nociceptors.
These free nerve endings are found closest to the surface of the skin when compared to other mechanoreceptors. Similar to other somatosensory mechanoreceptors, the sensory end of the pseudounipolar neurons have their cell soma in the dorsal root ganglion. When a nociceptor is activated, it causes the opening of mechanically-gated ion channels that allow for cation influx, resulting in cell depolarization.
Once depolarization occurs in response to a painful stimulus, action potential propagation depends on the action of voltage-gated sodium and potassium channels. Lidocaine, a common local anesthetic, dulls pain by blocking these voltage-gated sodium channels, preventing signal transmission. A special type of sodium channel, Na1.7, is present only in nociceptor fibers. A mutation in this ion channel that prevents it from functioning can results in the inability to feel pain.
Activation of nociceptors usually occurs in response to tissue damage or the threat of damage. Depending on the type of stimulus, either Aδ fibers or C fibers are activated to transmit information to the CNS. Nociceptors are located throughout the body in skin, muscles, and viscera, but there are no nociceptors in the CNS.
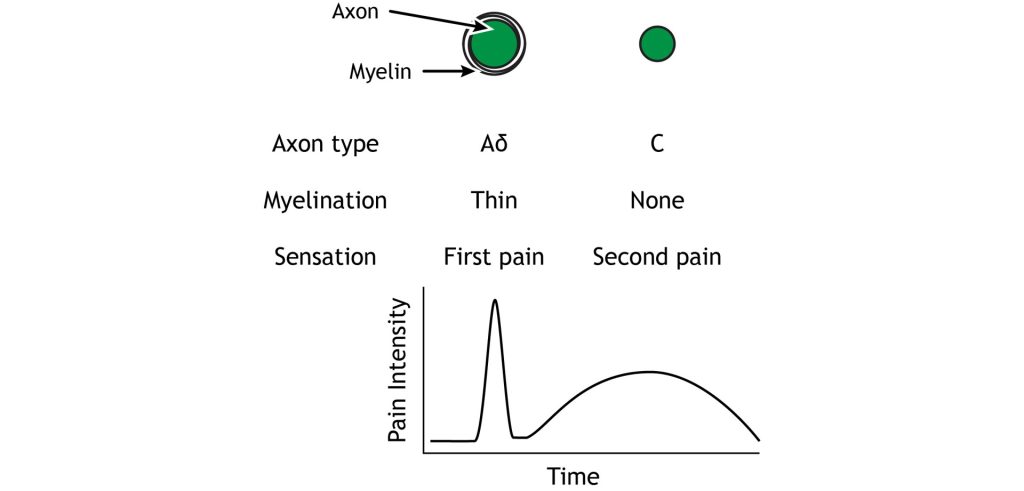
The Aδ fibers have small receptive fields which allows for precise location of pain. They are lightly myelinated, smaller in diameter (~5 μM), and transmit signals slower than the Aβ fibers that transmit touch information, around ~25 m/s. There are 2 types of Aδ fibers. Type Aδ I fibers have a low threshold for mechanical stimulation and activate following intense pressure or an incision on the skin. Type Aδ II fibers have a low threshold for thermal stimulation and activate in extremely hot or extremely cold environments.
Type C fibers tend to be polymodal and are activated by a range of stimuli including mechanical, chemical, and thermal. They have larger receptive fields, which results in a lower ability to locate pain. The unmyelinated C fibers have an even smaller diameter (~1 μm) and slower conduction velocity (~1 m/s) than the Aδ fibers. The C fibers carry upwards of 70% of noxious signals within the body.
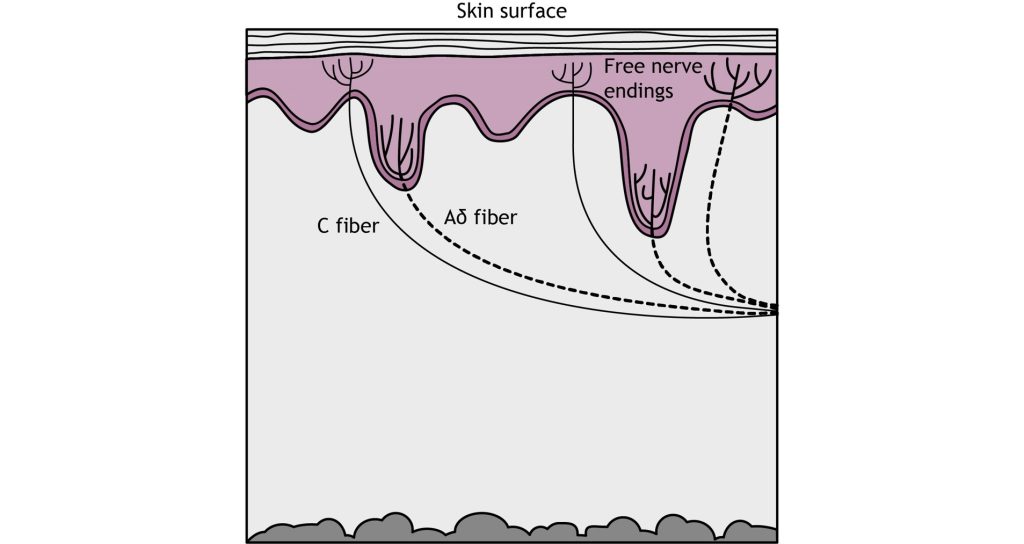
The Aδ and C fibers transmit information to the CNS at different speeds because of their different myelination levels. We can often perceive this difference in the form of “first pain” and “second pain”. Think about a time where you were injured, perhaps you hit your thumb with a hammer. Immediately, you might have felt an intense, sharp, and localized pain – first pain, but then a milder, burning or aching pain, that was more widespread that continue longer – second pain. The myelinated Aδ fibers are responsible for first pain transmission whereas the unmyelinated C fibers are responsible for second pain transmission.
Pathway to the Brain
Spinal Cord Branching
Primary afferent pain fibers have their cell bodies located in the dorsal root ganglion, like the fibers of the mechanoreceptors responsible for touch. The axons of these first-order neurons enter the ipsilateral dorsal side of the spinal cord, and then they branch and travel up and down the spinal cord a couple of segments in a white matter region of the spinal cord just posterior to the dorsal horn called Lissauer’s tract. All the branches terminate in the dorsal horn.
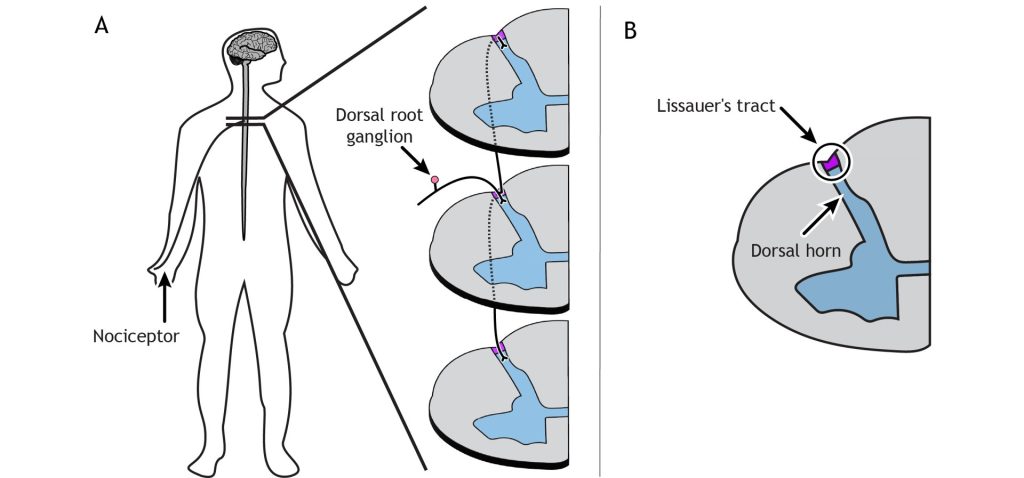
The primary pain afferents coming from the periphery release glutamate as their neurotransmitter. These afferents can also release the neuropeptide Substance P following periods of high stimulation.
Spinothalamic Pathway
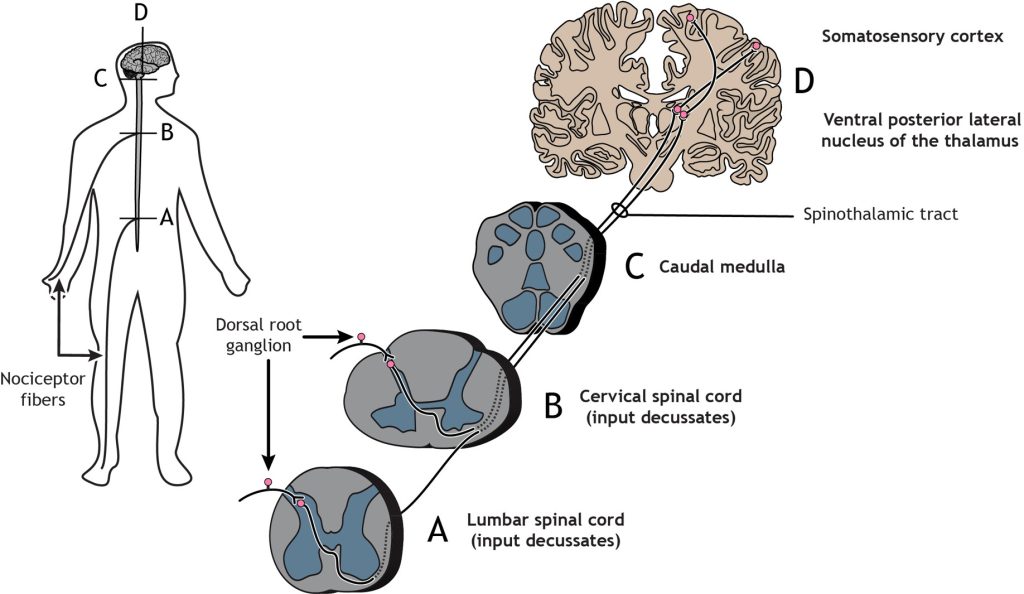
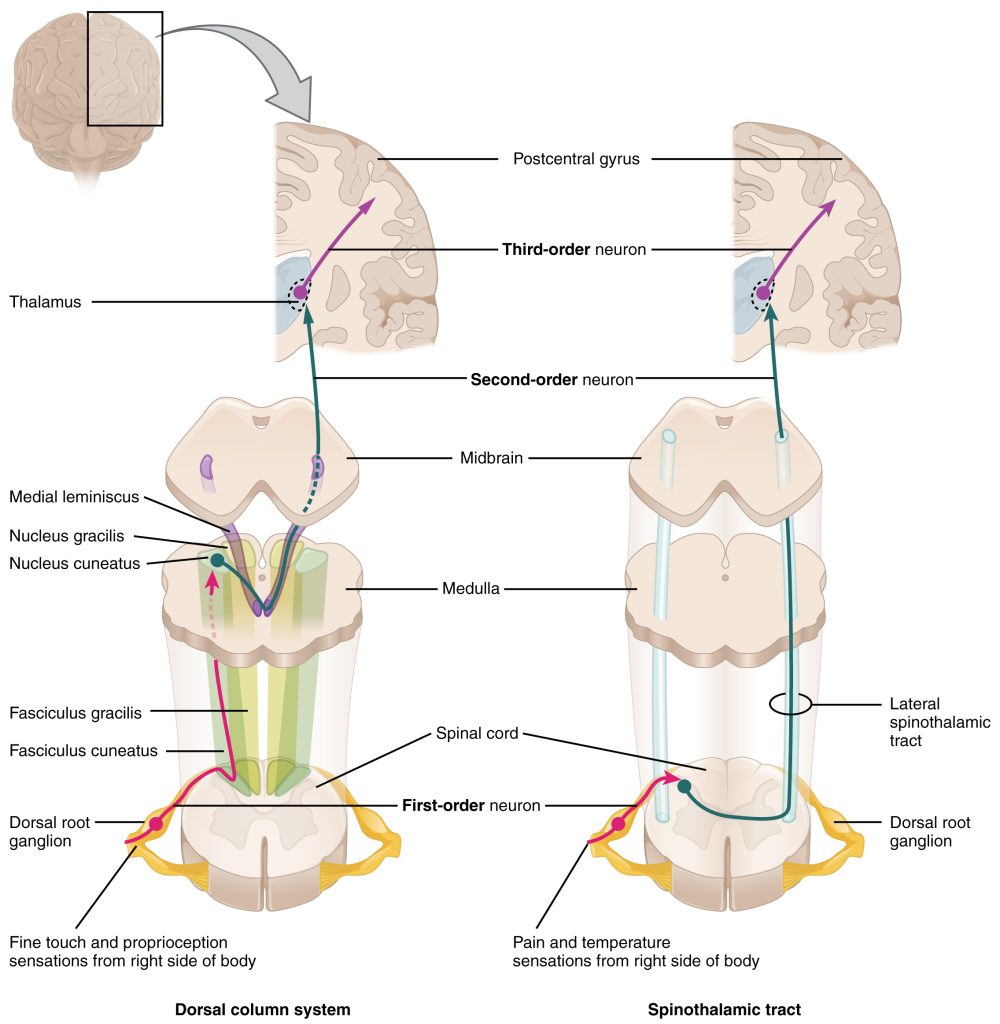
The nociceptor fibers (first-order sensory neurons) make synaptic contact with second-order neurons in the dorsal horn of the spinal cord. These neurons immediately cross the midline, or decussate, and then ascend to the brain through the anteriolateral aspect of the spinal cord and brainstem via the spinothalamic tract. The axons terminate in the ventral posterior lateral nucleus of the thalamus. The thalamic neurons (third-order neurons) then project to the primary somatosensory cortex located in the postcentral gyrus in the parietal lobe.
Like the touch pathway (Dorsal column medial lemniscus pathway), the pain pathway (spinothalamic pathway) is made up of three neurons that ultimately transmit the sensory information to the appropriate area of the cortex. The first order neuron is the sensory nociceptor neuron, the second order neuron is located within the spinal cord, and the third order neuron is located in the thalamus, that extends out to the primary somatosensory cortex.
Trigeminothalamic Pathway
Nociceptors in the face and head send info to the brain primarily through cranial nerve V, the trigeminal nerve. The first-order neurons have their cell bodies in the trigeminal ganglion, located just outside of the brainstem. The fibers enter the brainstem and descend to the spinal trigeminal nucleus in the medulla, where they synapse on a second-order neuron. The second-order neurons cross the midline and project up to the ventral posterior medial nucleus of the thalamus. These neurons then send projections to the face region of the somatosensory cortex.
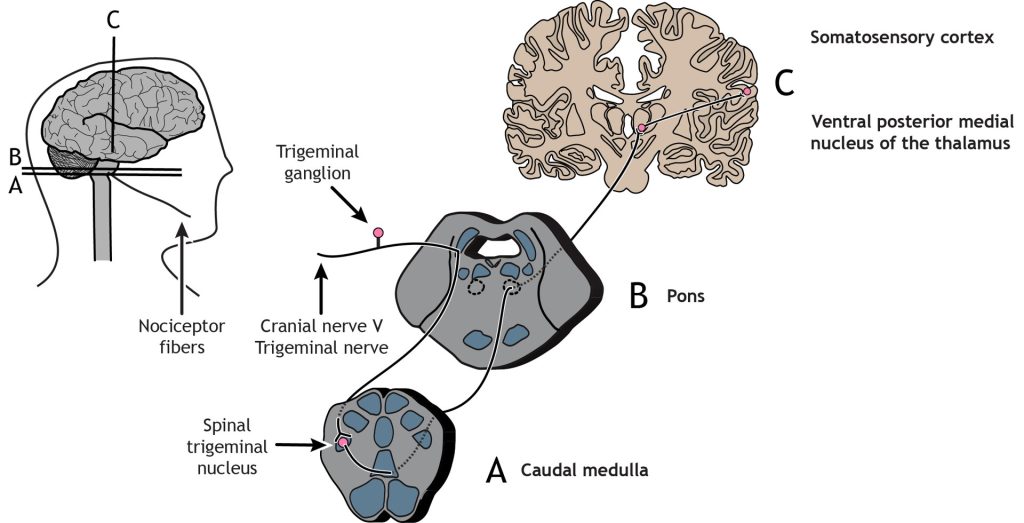
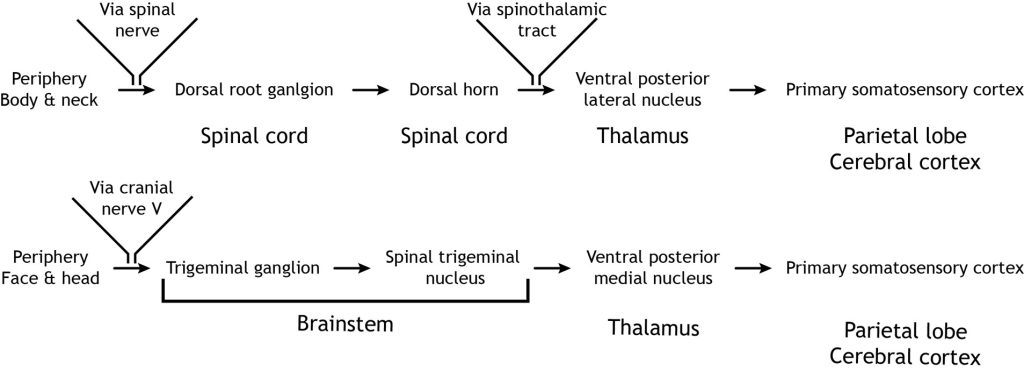
In summary, the touch pathway (Dorsal column medial lemniscus pathway) and the pain pathway (spinothalamic pathway) are made up of three neurons that ultimately transmit the sensory information between the periphery and the appropriate area of the cortex.
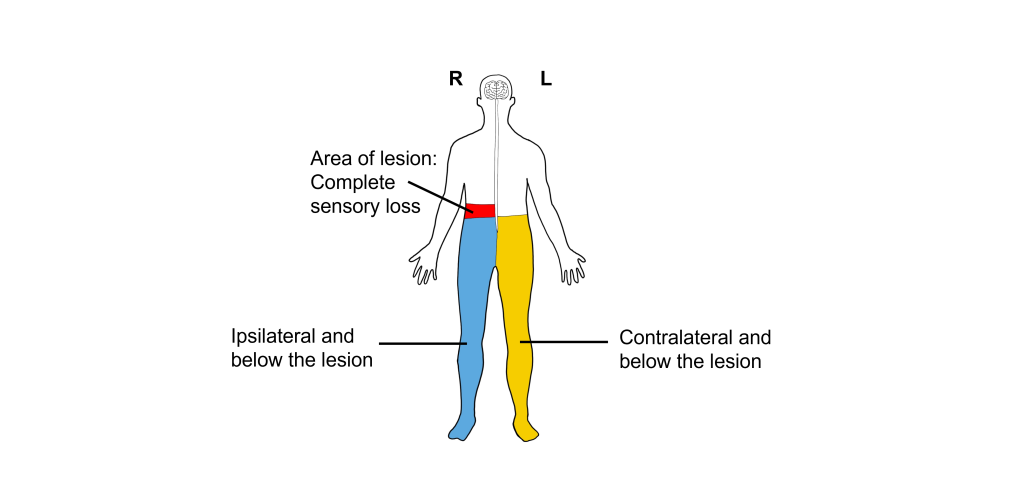
Dissociated Sensory Loss
Because the sensations of touch and pain decussate at different levels of the CNS as they ascend to the primary somatosensory cortex for processing, damage to only one half of the spinal cord will result in a condition referred to as dissociated sensory loss.
Ipsilateral to and below the damaged half of the spinal cord, individuals will have decreased touch sensation but normal pain sensation. Contralateral to and below the damaged half of the spinal cord, there will be normal touch sensation, but decreased pain sensation. Sensation above the damage will be unchanged.
Referred Pain
One of the warning signs that a person is having a heart attack is perception of pain in the shoulders or medial aspect of the left arm. This sensation is called referred pain: the feeling of pain at a site separate from where an injury is located. Referred pain happens when the nervous system is unclear about how to process signals from an internal organ, and the brain interprets those afferent signals as bodily pain. Injury at different internal organs cause different patterns of somatic pain: liver injury may present as pain in the shoulder blades, lung cancer sometimes causes shoulder pain, and kidney stones can cause pain in the lower back, abdomen, and sides. Even the headache-like pain you get from a “brain freeze” from drinking cold liquids too quickly is a form of referred pain.
It is unclear what causes referred pain. The convergence projection theory suggests that the afferent projections from the internal organs and the nociceptive somatosensory neurons of the skin form synapses onto the same population of spinothalamic tract secondary neurons. When these secondary neurons project towards the brain, there is no ability to differentiate the origin of the signal coming from the primary neuron.
The central sensitization theory suggests that the neurons of the spinal cord change in their excitability threshold with prolonged exposure to injurious stimuli. So, when an injured internal organ repeatedly sends signals into the secondary neurons, a host of neurotransmitters cause sensitization of the nociceptive signaling, which are now activated under non-noxious conditions.
Pain Regulation
Pain Disorders
In healthy individuals, nociceptors that detect pain send those signals into the spinal cord. But in people experiencing allodynia, noninjurious tactile stimuli, such as strands of hair brushing across your forehead or the feeling of a shirt resting on your shoulders, cause the sensation of pain. Diabetes, physical trauma, or postherpetic neuralgia may cause allodynia. Drugs that modify the action potential firing properties of the afferent somatosensory pathway, such as topical anesthetics (voltage-gated sodium channel inhibitors) or calcium channel antagonists, can help relieve the pain. A related disorder is hyperalgesia, an abnormally heightened perception and increased persistence of pain. When you experience a cut on your finger, and the area surrounding the cut is reddened and painful, that is the area of hyperalgesia. Both allodynia and hyperalgesia can be the result of sensitization, where repeated firing of the neurons increase action potential firing of pain neurons. Sensitization within this system can lead to functional and structural changes wherein weak stimuli that previously did not cause pain will now result in pain perception. Sensitization can occur in first-order, second-order, or third-order neurons within the pain pathway.
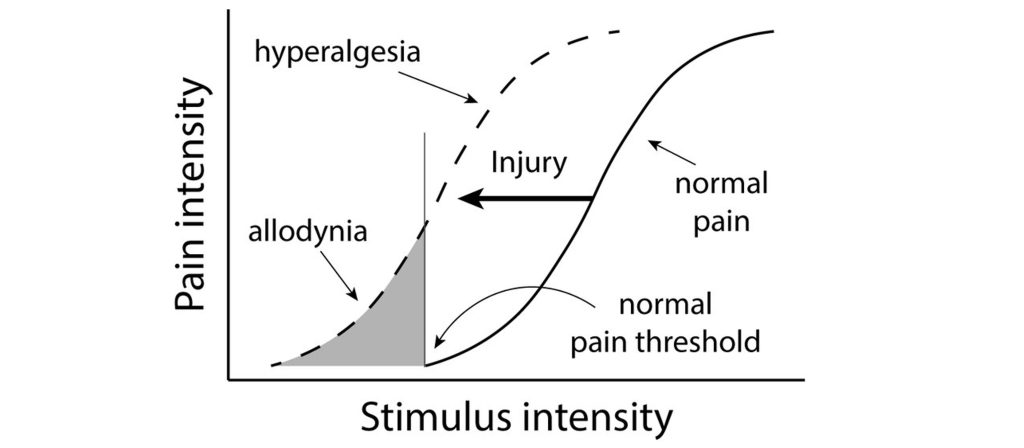
Allodynia and hyperalgesia can be symptoms of neuropathic pain, a broad category of pain conditions resulting from damage to the nervous system. People experiencing pain even in the absence of pain-producing stimuli may be diagnosed with chronic pain. Chronic pain may be localized or widespread, affecting large areas of the body at once. Up to 10% of the global population may experience chronic pain, and a variety of risk factors contribute, from advanced age, being male, low socioeconomic status, unhealthy lifestyles, and a history of surgical intervention. Chronic pain can lead to significantly shorter lifespans and may contribute to the opioid misuse epidemic.
A philosophically-opposite condition is called congenital insensitivity to pain (CIP). People with CIP are incapable of perceiving pain, no matter how severe. CIP is a rare genetic condition that is observed from birth. People with CIP have significantly shorter lifespans since they do not sense that their bodies may be injured and may not learn to avoid exposure to damaging stimuli. Some patients may walk for days on a compound fracture of the shin bone, or never even notice that their back is broken. CIP is associated with mutations in the SCN9A gene, which codes for a component of the voltage-gated sodium channels that are expressed in nociceptors.
Descending Pain Regulation- Gate Control Theory
Individuals can have horrible injuries, yet not experience any pain in the moment. How can this occur?
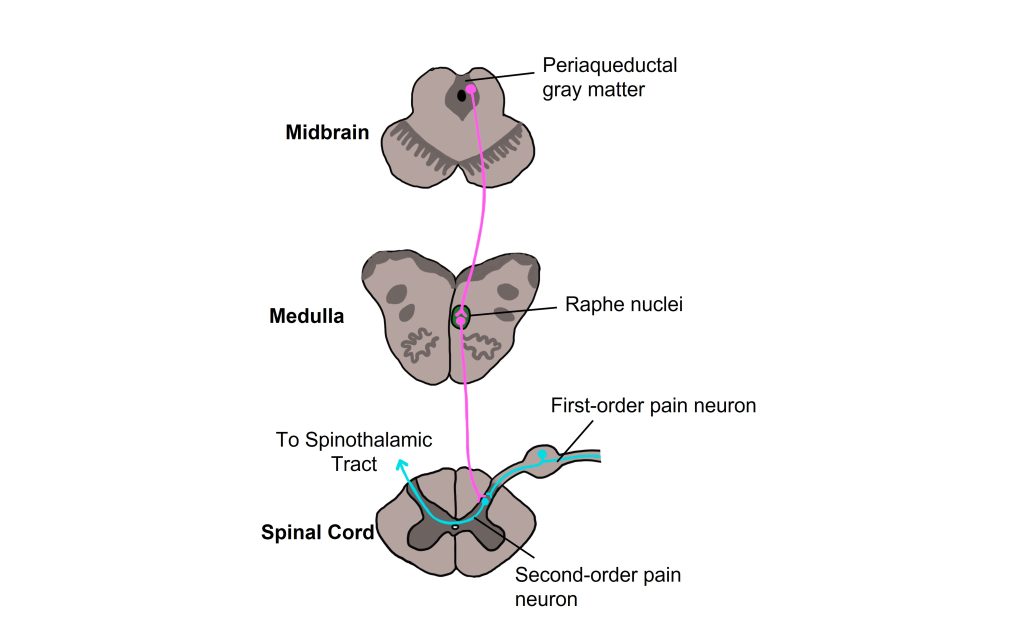
One theory for how this is accomplished is ‘The Gate Control Theory of pain modulation’ which postulates that there are descending pathways that start in the brain and signal to the spinal cord that acts to inhibit, or “gate”, the pain signals from ascending to the brain and being perceived as pain. One brain area implicated in this pathway is the periaqueductal gray (PAG) matter within the midbrain. Neurons with their cell bodies within the PAG have axons that descend to the Raphe nuclei within the medulla. The raphe nuclei neurons then descend the spinal cord and release serotonin onto the second-order spinal pain neuron. The second-order pain neuron is therefore receiving synapses from both the first-order pain neuron that is transmitting the pain signal from the periphery and the raphe nuclei neuron carrying the descending signal from the CNS. The serotonin released by the raphe nuclei neuron acts to inhibit activity of the second-order pain neuron, thereby inhibiting the pain signal from ascending to the brain. In what situations do the PAG neurons ‘gate’ the pain signals from ascending to the brain and being perceived as pain? That answer is less clear, though we do understand that the gating of the pain signal eventually stops and the pain is able to be perceived.
Endogenous Opioids
The experience of pain can be altered through drugs that interact with the brain. An analgesic decreases pain without a loss of consciousness, whereas central anesthetics cause a loss of consciousness and completely eliminate all incoming sensory experiences, including pain and somatosensory information.
Opioids are drugs that are very effective at short-term pain relief. They are derived from the poppy plant and include opium, morphine, codeine, heroin, and analogues of those substances. Opioids bind strongly to several types of opioid receptors (all G-protein-coupled receptors) that are located throughout the brain, with especially high receptor density in areas such as the PAG, raphe nuclei, and in the spinal cord. Antagonists for opioid receptors block the effects of opioids and include drugs like naloxone and naltrexone.
The brain also makes its own endogenous opioids including endorphins, enkephalins, and dynorphins, that bind to these same receptors within the brain. Endogenous opioids are involved in various functions within the brain including nociception, respiration, cardiovascular, neuroendocrine, food intake, learning and memory, and many others. When opioids (either exogenous or endogenous) bind to their receptors, they modulate incoming pain information and provide temporary pain relief. Opioids are known to have effects on mood and can produce euphoric feelings. Unfortunately, they also have several side effects, most notably drug tolerance and dependence.
Phantom Limb Phenomenon
Individuals that have undergone amputation of a limb can experience ‘phantom limbs’, which give the sensory experience that the amputated limb is still present. In these cases, the sensory neurons can no longer provide sensory or pain information from the amputated limb, yet somatosensory and pain perception persists. Some individuals that experience phantom limbs can also experience phantom limb pain, where pain is experienced in a limb that has been removed.
Many physicians believed that phantom limbs were caused by irritation of nerve endings that remained in the limb stump after amputation. However, the research by the neuroscientist Dr. V.S. Ramachandran demonstrated that cortical plasticity played a major role in producing phantom limbs. Specifically, Dr. Ramachandran believed that cortical plasticity within the somatosensory cortex was responsible for the sensation of phantom limbs. He hypothesized that adjacent somatosensory maps within the cortex blurred together following amputation, causing the brain to rewire in the absence of incoming sensory information.
The following case study demonstrated this somatosensory reorganization following amputation. Dr. Ramachandran worked with an individual named Tom Sorenson that had lost an arm in a car accident. Tom was experiencing an itch on his phantom arm that he could not scratch. Due to the fact that the cortical maps for the face and the arm are adjacent to each other within the somatosensory cortex, Ramachandran methodically used a swab to stroke different areas of Tom’s face. Tom reported that in certain areas of his face, he could also feel the swab on his phantom limb. Functional brain scans confirmed that the maps for the face and the arm had blurred together.
Ramachandran believes that phantom limb pain stems from a “learned paralysis” that occurs when an individual sends out a motor command to move a phantom limb but receives no proprioceptive or visual confirmation that the phantom limb has moved. After this experience is repeated, the brain wires in such a way that it learns that the phantom limb does not move, leading to feelings of paralysis and pain associated with the phantom limb. To treat this “learned paralysis” in the phantom, Ramachandran developed mirror box therapy. In mirror box therapy, an amputee uses a mirror to produce a reflection of the intact limb opposite the amputated limb. By looking at this reflection, the individual experiences visual feedback that the amputated limb is intact and can now move. Looking at the movement of the “phantom” limb in the mirror, provides powerful input to the nervous system and acts to alleviate the feelings of paralysis and pain associated with the phantom limb, and effectively “unlearn” the paralysis and feelings of pain.

Key Takeaways
- Nociceptors are free nerve endings located throughout the body.
- Pain information is carried on Aδ and C afferent fibers that are separately responsible for first and second pain, respectfully.
- The spinothalamic pathway is a three neuron path that carries pain information from the body to the somatosensory cortex and the trigeminothalamic pathway carries pain information from the head and face to the somatosensory cortex.
- Dissociated sensory loss results from damage to one half of the spinal cord.
- Referred pain is the feeling of pain at a site separate from where an injury is located.
- Allodynia and hyperalgesia are disorders of pain perception and can occur following sensitization of neurons within the pain pathway.
- Descending pathways from the brain can modulate pain perception.
- The opioid system within the body is important in modulating pain information.
- Phantom limb pain can occur in individuals with an amputated limb. Mirror box therapy can treat those with phantom limb pain.
Attributions
Portions of this chapter were remixed and revised from the following sources:
- Foundations of Neuroscience by Casey Henley. The original work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
- Open Neuroscience Initiative by Austin Lim. The original work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Media Attributions
- Nociceptors © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Pain fibers © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Lissauer’s tract © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Pain Pathway from Body © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Pain Pathway from Face © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Pain Pathways © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Touch and Pain pathways © OpenStax College adapted by Valerie Hedges is licensed under a CC BY (Attribution) license
- Dissociated sensory loss © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Referred Pain Chart © Open Stax College is licensed under a CC BY (Attribution) license
- Pain Intensity © Richard Lennertz adapted by Valerie Hedges is licensed under a CC BY (Attribution) license
- Descending pain regulation © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Phantom limb mirror box © Golan Levin adapted by Valerie Hedges is licensed under a CC BY (Attribution) license
Sensory process that provides signals that trigger pain when in the presence of a noxious stimulus
receptors that sense noxious (painful) stimuli
a structure found outside the dorsal aspect of the spinal cord where sensory neuron cell bodies are located
occurs on the same side of the body
Toward the top of the brain or the back of the spinal cord
Behind; toward the back
cross the midline
Toward the bottom of the brain or the front of the spinal cord
Toward the edge
denotes the side of the body opposite to the side where a structure started
the feeling of pain at a site separate from where an injury is located
traveling towards the CNS
something that decreases pain

