49 Motivated Behavior: Reward Pathway
Having a sense of reward is a valuable adaptive trait. Consider two similar organisms living in the distant geologic past, both involved in Darwin’s struggle to pass their genetic material to the following generation. One of these creatures gets an internal rewarding sensation whenever it drinks water while thirsty, eats high-caloric-content food, or successfully reproduces. As can be expected, this organism is highly motivated to seek out those rewarding stimuli in the world. It is are more likely to crave sugary foods to maximize daily caloric intake necessary for activity and growth. The other creature does not experience pleasure from its actions, resulting in little motivation to seek out calories or to reproduce, for example. It stands to reason that the first of these two creatures has an evolutionary advantage to survive. As can be expected, the regions of the brain that are concerned with reward are highly conserved through evolution.
Reward Circuit
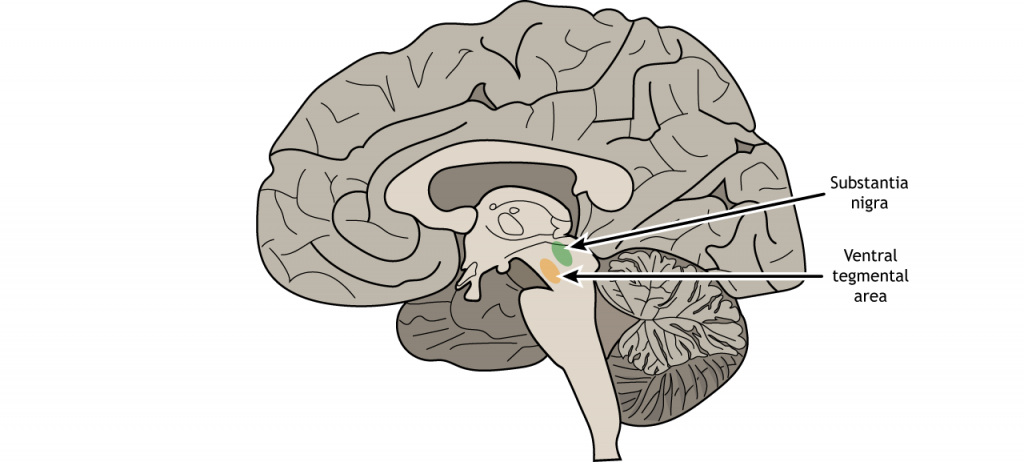
For us humans, our reward signaling neural circuitry starts with the ventral tegmental area (VTA). Located in the midbrain, the VTA contains neurons that synthesize the neurotransmitter dopamine (DA). This population of DA cells is similar to the cells in the substantia nigra, the midbrain neurons that are lost in Parkinson’s disease.
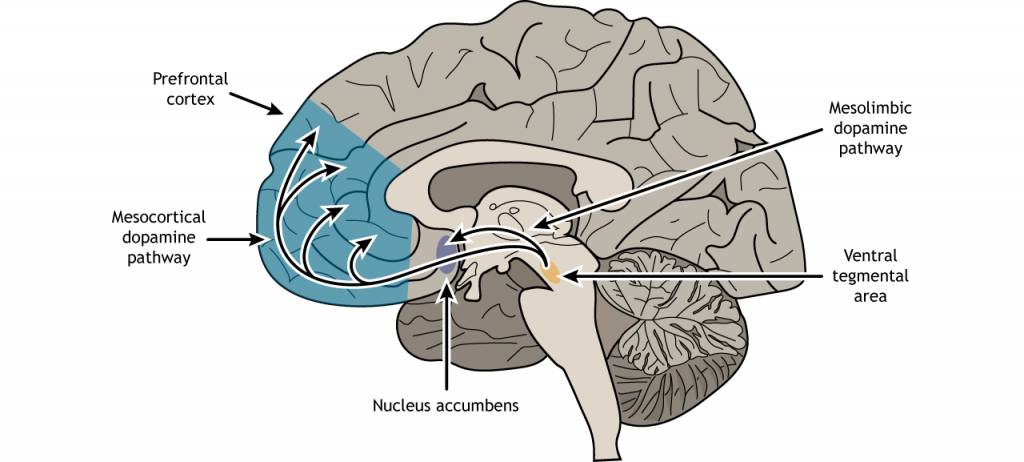
There are two primary pathways from the VTA that are important for reward.
The mesolimbic pathway consists of dopamine-producing neurons that release dopamine onto the cells in the nucleus accumbens (NAc; also sometimes called the ventral striatum). This seems to be the major pathway by which reward is mediated by the brain. Using microdialysis, it is possible to measure the dopamine concentration in the NAc during performance of some behavioral tasks. In studies with rats, engaging in rewarding activities such as eating or sex results in increased release of dopamine in the NAc. Similarly we see that release of dopamine into the human NAc is enhanced when we engage in activities we enjoy: binge eating sugary stuff, having sex, even playing video games. Notably, many drugs of abuse such as cocaine or amphetamine can induce a similar overflow of dopamine release when an animal is exposed to the drug.
The second area that receives VTA innervation is the prefrontal cortex (PFC), and this projection is called the mesocortical pathway. We think of the PFC as being involved in the conscious, decision making and inhibition of action.
View the amygdala using the BrainFacts.org 3D Brain
View the amygdala using the BrainFacts.org 3D Brain
One of the earliest experiments demonstrating functional evidence of a “reward circuit” was conducted at McGill University in the early 1950’s. Drs. James Olds and Peter Milner carried out surgery on anesthetized rats to implant metal electrodes into different areas of the brain. After recovery, the rats were placed into an operant conditioning chamber, also called a Skinner box. These special cages are equipped with a device which the subject can physically manipulate. For example, the cage may have a lever which the animal can push, or a hole into which they can poke their nose.
In the case of Olds and Milner’s experiments, a lever press led to activation of the implanted electrodes, resulting in neuronal activation. They had found that when the electrode was implanted into brain areas such as the corpus callosum or the hippocampus, the rats did not spend significant time pressing on the lever. However, the rats which had the electrodes implanted in the medial forebrain bundle that is located in the VTA to NAc pathway, responded frequently on the lever. One such rat pressed the lever almost 2000 times in less than an hour, averaging nearly one response every two seconds! Electrical activation of reward centers is so desirable, rats will choose electrical stimulation over food even to the point of starvation. This experimental paradigm is also called intracranial-self stimulation (ICSS). Treatment with drugs that block the receptors for dopamine reduce the self-stimulating behavior, indicating that dopamine is the critical neurotransmitter involved in making the stimulation of these brain regions rewarding.
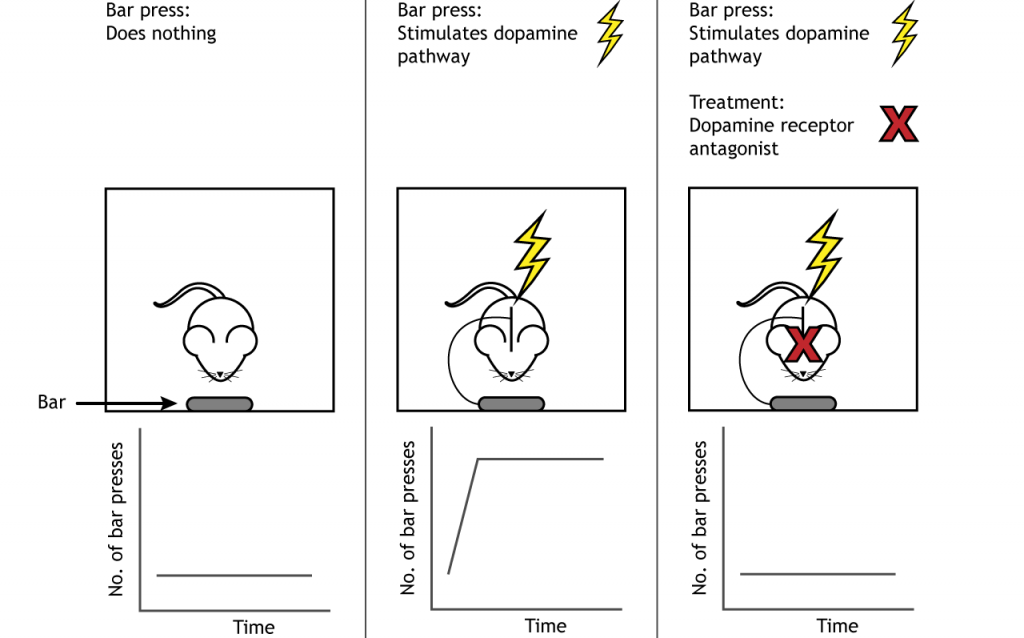
And while the original studies were conducted on rats, clinicians soon after tested their model in humans, with similar indwelling electrodes in different brain regions. Unsurprisingly, humans also have areas of the brain that are responsible for encoding reward, and when given the chance to electrically activate those areas, they do so extensively. When the experimenters brought in a tray of delicious food to the test chamber, the hungry patients—who hadn’t eaten for upwards of seven hours—simply looked at the meal, but couldn’t pull themselves away from the stimulation button long enough to eat. Artificial stimulation of these reward centers of the brain was more valuable to the patients than nutrition.
However, continued research suggests the connection between dopamine release and reward may not be as simple as the self-stimulation studies imply. It appears that it is not the reward itself that increases dopamine, but the predicted expectation of the reward . Dopamine signaling increases during anticipation of a predicted reward. If the level of reward is more than predicted, reward learning occurs, and dopamine signaling and motivation to repeat that behavior increases. If the level of reward is less than predicted, then dopamine signaling decreases as does motivation to repeat the behavior.
Rewarding Stimuli
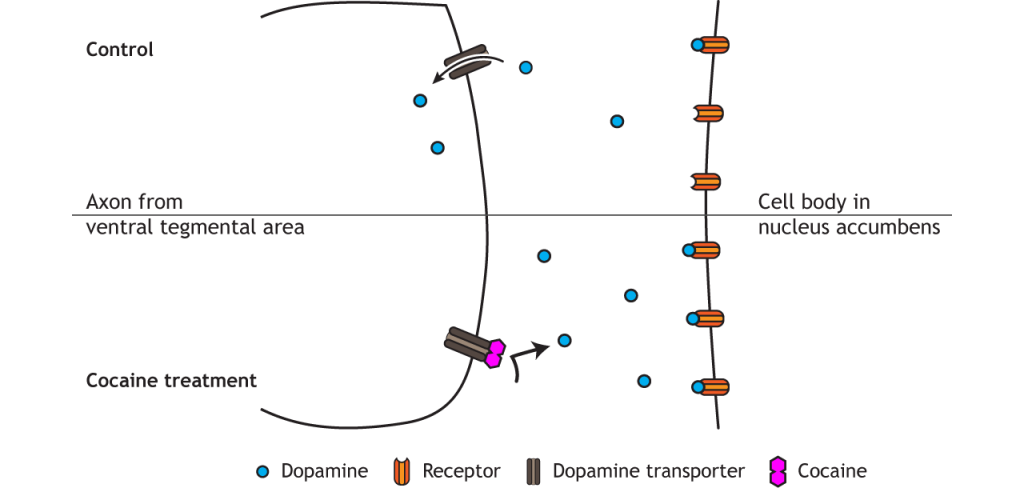
Natural rewards that increase survival and fitness of a species activate the reward circuit. These behaviors and stimuli include certain food (like those containing high sugar or fat levels), social bonding, parental bonding, and sex. Most drugs of abuse also activate the reward circuit and dopamine signaling, which plays a critical role in the formation of addiction. For example, cocaine blocks dopamine reuptake into presynaptic VTA terminals; heroin and nicotine increase dopamine release from the VTA. These alterations increase dopamine effect on neurons in the nucleus accumbens.
Key Takeaways
- The reward circuit involves dopamine release from the ventral tegmental area into the nucleus accumbens and prefrontal cortex.
- Self-stimulation experiments demonstrate the role of dopamine and the reward circuit.
- Dopamine signaling likely predicts reward value and can be altered if predicted outcomes differ from actual outcomes.
- Drugs of abuse act upon the reward circuit.
Attributions
Portions of this chapter were remixed and revised from the following sources:
- Foundations of Neuroscience by Casey Henley. The original work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
- Open Neuroscience Initiative by Austin Lim. The original work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Media Attributions
- Ventral Tegmental Area © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Mesolimbic Mesocortical Pathways © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- DopaminePathwayStimulation © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Cocaine Effect © Casey Henley is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
Neurological disorder of motor function resulting from the loss of dopamine-producing cells in the substantia nigra
dopamine producing neurons that start in the ventral tegmental area and project to the nucleus accumbens
dopaminergic neurons that start in the ventral tegmental area that project to the prefrontal cortex

