19 Neurotransmitters: Atypical Neurotransmitters
Although we generally think of
neurotransmitters as neurochemicals that
function as described above, there are a
few atypical neurotransmitters that don’t
quite fit the mold of the other chemical
signals.
Neuropeptides
Neuropeptides are a class of large signaling molecules that some neurons synthesize. Neuropeptides are different from the traditional neurotransmitters because of their chemical size. Neuropeptides are a short string of amino acids and are known to have a wide range of effects from emotions to pain perception. Unlike small molecule neurotransmitters, neuropeptides are synthesized in the cell body and transported to the axon terminal.
Synthesis and Storage
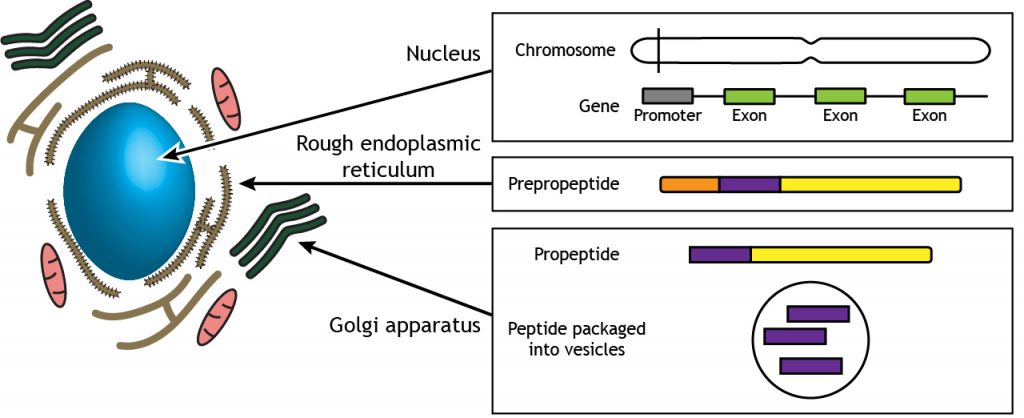
Like other proteins, neuropeptides are synthesized from mRNA into peptide chains made from amino acids. In most cases, a larger precursor molecule called the prepropeptide is translated into the original amino acid sequence in the rough endoplasmic reticulum. The prepropeptide is processed further to the propeptide stage. The remaining processing and packaging of the final neuropeptide into a vesicle occurs in the Golgi apparatus.
Monoamines like dopamine, norepinephrine, or serotonin have a molecular weight around 150-200, while one of the smaller neuropeptides, enkephalin, has a molecular weight of 570. One of the largest neuropeptides, dynorphin, has a molecular weight greater than 2,000. Because of their large size, neuropeptides have to be packaged in dense core vesicles very close to the site of production near the nucleus, rather than in clear vesicles right at the terminal. These large vesicles must then move from the soma to the terminal.
Figure 19.1. Neuropeptide synthesis occurs in the cell body. Each neuropeptide is encoded by a gene on the DNA located in the nucleus. mRNA is translated into an amino acid sequence for a precursor molecule called a prepropeptide in the rough endoplasmic reticulum. Further processing and packaging of the neuropeptide into vesicles occurs in the Golgi apparatus. ‘Neuropeptide Synthesis’ by Casey Henley is licensed under a Creative Commons Attribution Non-Commercial Share-Alike (CC BY-NC-SA) 4.0 International License.
Axonal Transport of Neuropeptides
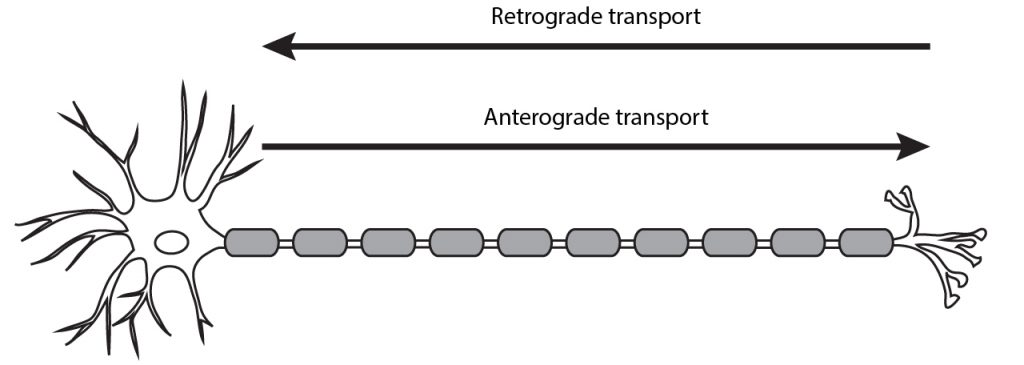
The packaged peptides need to be transported to the presynaptic terminals to be released into the synaptic cleft. Organelles, vesicles, and proteins can be moved from the cell body to the terminal via anterograde transport or from the terminal to the cell body via retrograde transport. Anterograde transport can be either fast or slow.
The packaged neuropeptides are transported to the synaptic terminals via fast anterograde axonal transport mechanisms.
Neuropeptide Receptors
Neuropeptides such as enkephalin and dynorphin are agonists at a class of receptors called the opioid receptors. These opioid receptors fall into four main types. The three classical opioid receptors are named using Greek letters: δ (delta), μ (mu), and κ (kappa); the fourth class is the nociceptin receptor. All of these receptors are inhibitory metabotropic receptors which signal using the Gαi protein. These receptors are expressed in several brain areas, but expression is particularly heavy in the periaqueductal gray, a midbrain area that functions to inhibit the sensation of pain. Drugs that activate the opioid receptors, like morphine, oxycontin, or fentanyl, are the most effective clinical treatments that we know of for acute pain. Unfortunately, these same drugs also represent a tremendous health risk, as opioid drugs can be lethal in overdose and have a high risk of substance use disorder.
Endocannabinoids
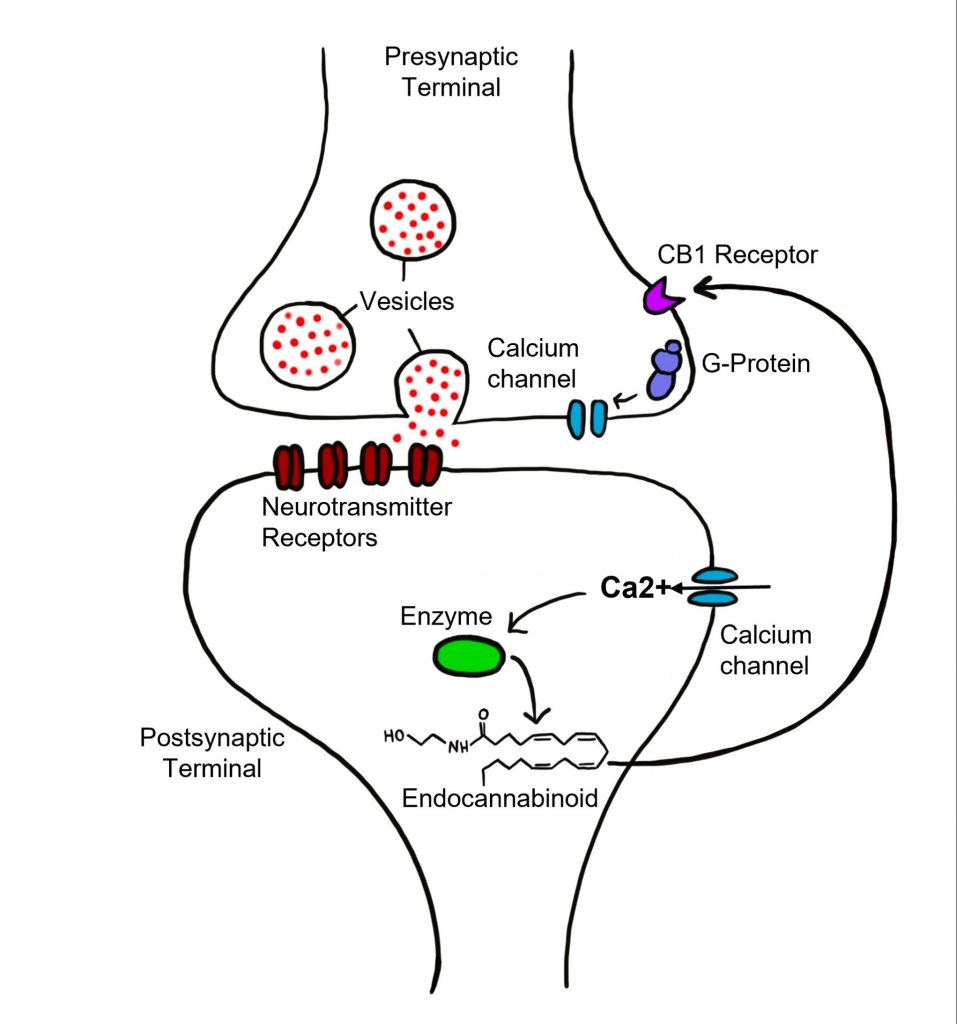
Endocannabinoids are endogenous lipid neurotransmitters that are a bit unusual compared to the other neurotransmitters that we have covered. First, they signal in a retrograde manner. This means that the endocannabinoids neurotransmitters are released by the postsynaptic cell and bind to receptors on the presynaptic cell. Additionally, endocannabinoids are not packaged into vesicles and released by fusion with the cell membrane.
Instead, when the postsynaptic cell has an action potential, it causes voltage-gated calcium channels in the postsynaptic cell to open, and for calcium ions to rush into the postsynaptic cell. The local increase in calcium concentration within the postsynaptic cell triggers the cell to synthesize endocannabinoids. Again, these neurotransmitters are not stored in vesicles like the other neurotransmitters that have been discussed. Instead, these lipid neurotransmitters are made on demand, or synthesized ‘de novo’. As lipids, the endocannabinoids are membrane permeable and can diffuse out of the cell through the cell membrane. The two most well-characterized eCBs in humans are called 2-Arachidonoylglycerol (2-AG) and Anandamide (ANA).
The endocannabinoids bind to one of two receptors, CB1 or CB2. Both receptor types are inhibitory metabotropic receptors that are located on the presynaptic cell and coupled to Gαi. Generally, CB1 receptors are found in the nervous system, while the CB2 receptors are found elsewhere in the body, such as in the immune system. Activity of the metabotropic CB1 receptors causes presynaptic calcium channels to close, reducing the concentration of calcium in the presynaptic cell, and thus decreasing the amount of neurotransmitter released by the presynaptic cell.
The endocannabinoid system is widely used by various systems in the body. It is estimated that endocannabinoid receptors are the most abundant GPCRs in the whole body. These substances were named because they are endogenous chemicals that are functionally similar to compounds found in plants of the genus Cannabis. One reason cannabis is used is because of its ability to interact with our endocannabinoid receptors.
Nitric Oxide
The nervous system is capable of signaling via the gas nitric oxide (NO). This gas transmitter is not stored in vesicles, but is synthesized as it is needed. NO is formed when the amino acid arginine is degraded by the enzyme NO synthase (NOS). Because NO is a gas, it easily permeates across cell membranes. Therefore, the receptors for NO do not need to be transmembrane proteins expressed on the cell surface. Instead, the receptor for NO is an intracellular receptor called soluble guanylate cyclase (sGC). SGC works through a signaling pathway that is different from other metabotropic receptors so far described. SGC is linked with the signaling molecule cyclic GMP (cGMP), which activates protein kinase G (PKG). PKG can either be excitatory or inhibitory, depending on the intracellular components.
Key Takeaways
- Neuropeptides are proteins that are synthesized in the cell body and are transported in vesicles to the axon terminal
- Endocannabinoids are lipid neurotransmitters that use retrograde signaling. They are synthesized and released by the postsynaptic cell and bind to metabotropic CB1 receptors on the presynaptic cell to alter neurotransmitter release.
- Nitric oxide is a gas neurotransmitter that is not stored in vesicles but instead is made on demand. As a gas, nitric oxide can easily cross the phospholipid bilayer and bind to intracellular receptors.
Test Yourself!
Attributions
Portions of this chapter were remixed and revised from the following sources:
- Foundations of Neuroscience by Casey Henley. The original work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
- Open Neuroscience Initiative by Austin Lim. The original work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Media Attributions
- Untitled_Artwork 2
Movement from the cell body to the axon terminals
Movement from the axon terminals toward the cell body
made within the body
Ion channels that open/close in response to changes in voltage (membrane charge)
allows for passage across the cell membrane
Postsynaptic receptors in which neurotransmitters bind and cause the activation of an associated G-protein and cell signaling cascades. The affected ion channel and receptor are found on different transmembrane proteins..