26 Pain
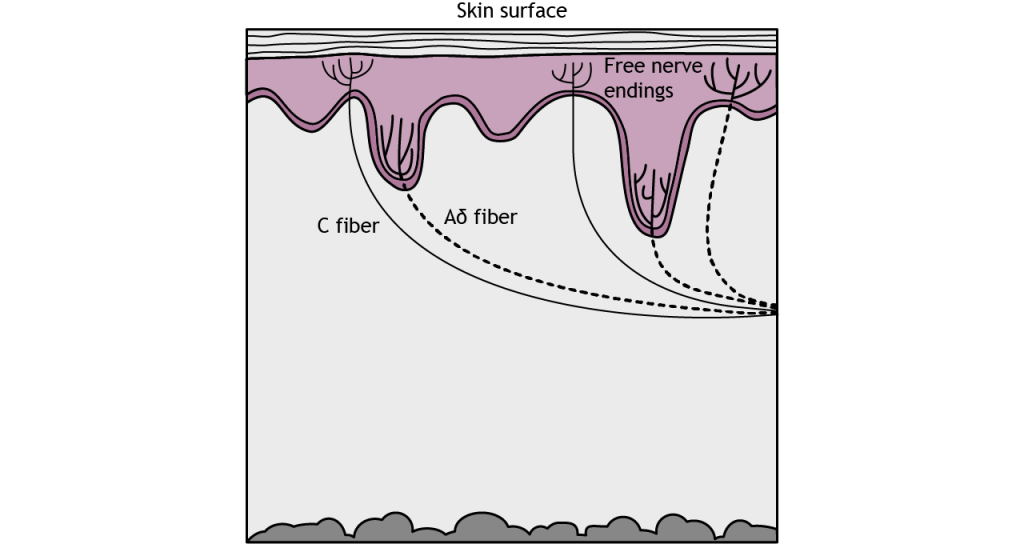
Pain is the sensation we feel in response to activation of special branching, bare nerve endings called nociceptors. Activation of nociceptors usually occurs in response to tissue damage or the threat of damage. Depending on the type of stimulus, either lightly myelinated A delta fibers or unmyelinated C fibers transmit information to the CNS. Nociceptors are located throughout the body in skin, muscles, and viscera, but nociceptors are sparse in most CNS tissue.
Receptors
The type of pain perceived depends on which receptor type is activated.
There are three types of nociceptors that are each activated by different types of harmful stimuli. Type A delta I fibers have a low threshold for mechanical stimulation, such as intense pressure or an incision on the skin. Type A delta II fibers have a low threshold for thermal stimulation and activate in extremely hot or extremely cold environments. Type C fibers tend to be polymodal or activated by a range of stimuli including mechanical, chemical, and thermal.
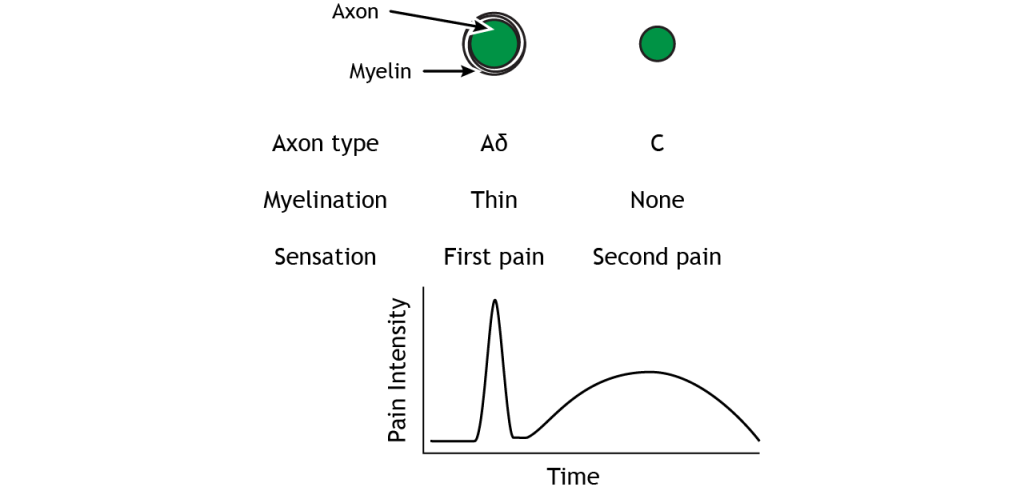
The A delta and C fibers transmit information to the CNS at different speeds because of their different myelination levels. We can often perceive this difference in the form of “first pain” and “second pain”. Think about a time where you were injured, perhaps you hit your thumb with a hammer. Immediately, you might have felt an intense, sharp pain – first pain, but then a milder, burning or aching pain that continue longer – second pain. A delta fibers are responsible for first pain transmission whereas C fibers are responsible for second pain transmission.
Pain Transduction
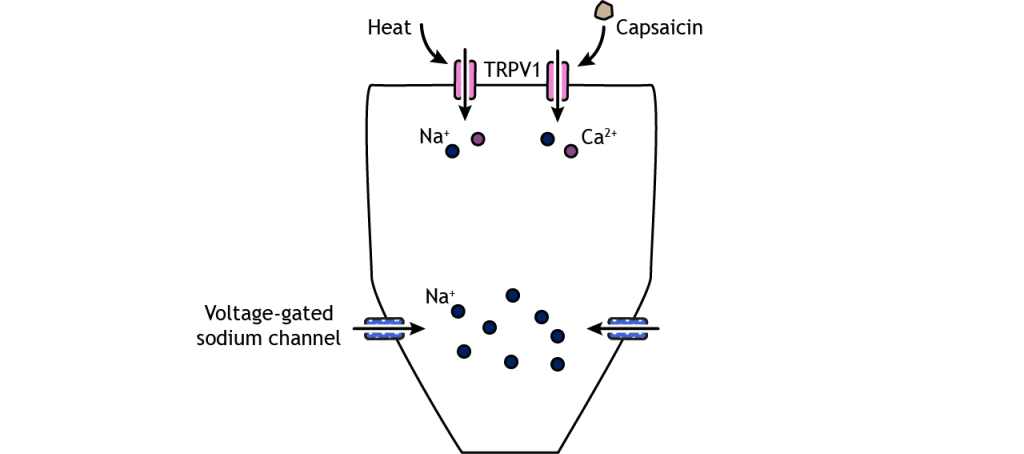
Like the other sensory systems, specialized proteins in the cell membrane of nociceptors convert noxious stimuli into electrical potentials in the neurons. A family of ion channels called transient receptor potential (TRP) channels can be expressed in the nociceptor nerve endings and have been shown to be activated by thermal and chemical components. For example, TRPV1, a non-selective cation channel found in nociceptors, can be activated by temperatures above 45 degrees C and can also be activated by a chemical in hot peppers called capsaicin. Another family of receptors called Piezo is believed to be responsible for some mechanical pain transduction.
Once depolarization occurs in response to a painful stimulus, action potential propagation depends on the action of voltage-gated sodium and potassium channels. Lidocaine, a common local anesthetic, dulls pain by blocking these voltage-gated sodium channels, preventing signal transmission. A special type of sodium channel, Na1.7, is present only in nociceptor fibers. A mutation in this ion channel that prevents it from functioning can results in the inability to feel pain.
Pathway to Brain
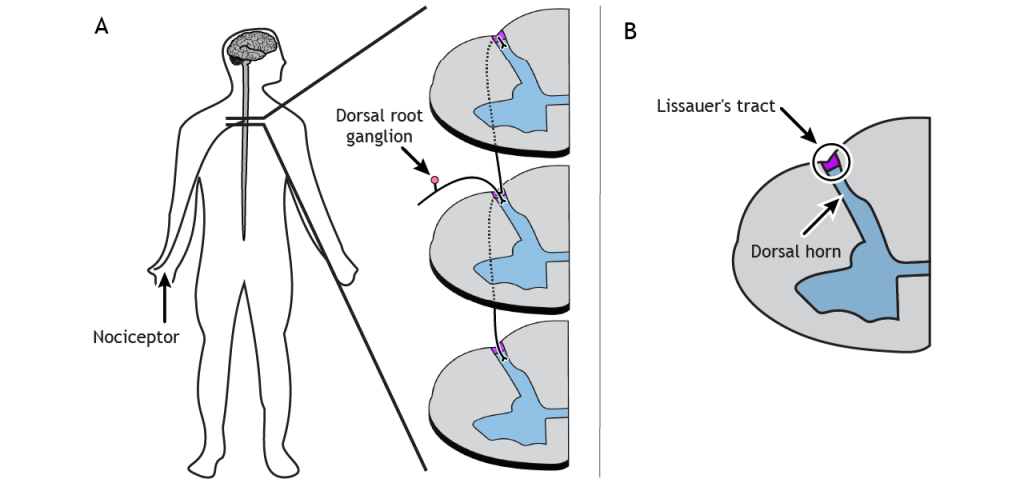
Spinal Cord Branching
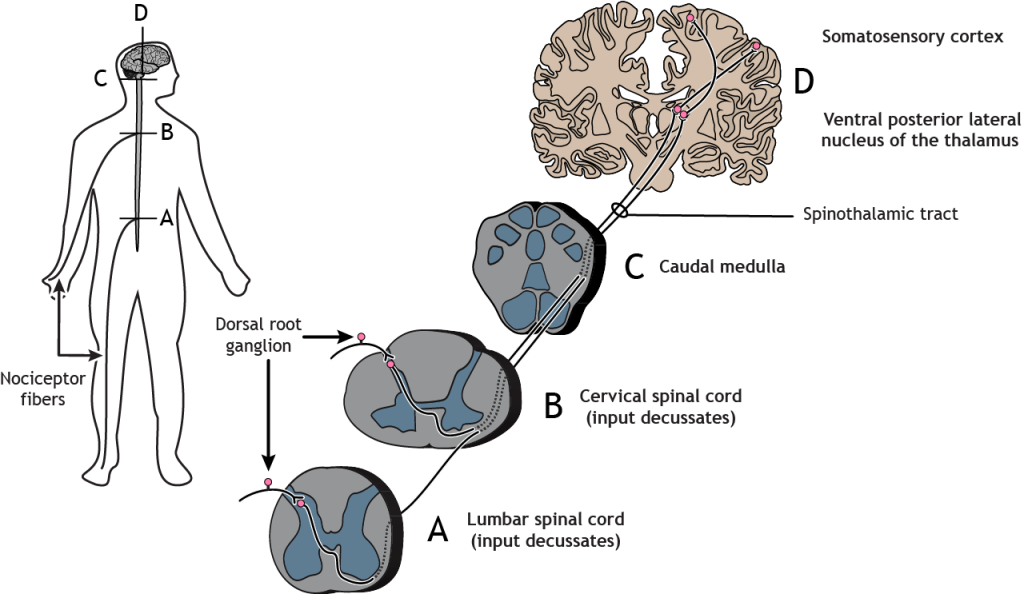
Primary afferent pain fibers have their cell bodies located in the dorsal root ganglion, like the fibers of the mechanoreceptors responsible for touch. The axons of these first-order neurons enter the ipsilateral dorsal side of the spinal cord, and then they branch and travel up and down the spinal cord a couple of segments in a white matter region of the spinal cord just posterior to the dorsal horn called Lissauer’s tract. All the branches terminate in the dorsal horn.
Spinothalamic Pathway
The nociceptor fibers make synaptic contact with second-order neurons in the dorsal horn of the spinal cord. These neurons immediately cross the midline, or decussate, and then ascend to the brain through the anteriolateral aspect of the spinal cord and brainstem via the spinothalamic tract. The axons terminate in the ventral posterior lateral nucleus of the thalamus. The thalamic neurons then project to the primary somatosensory cortex located in the postcentral gyrus in the parietal lobe.
Trigeminothalamic Pathway
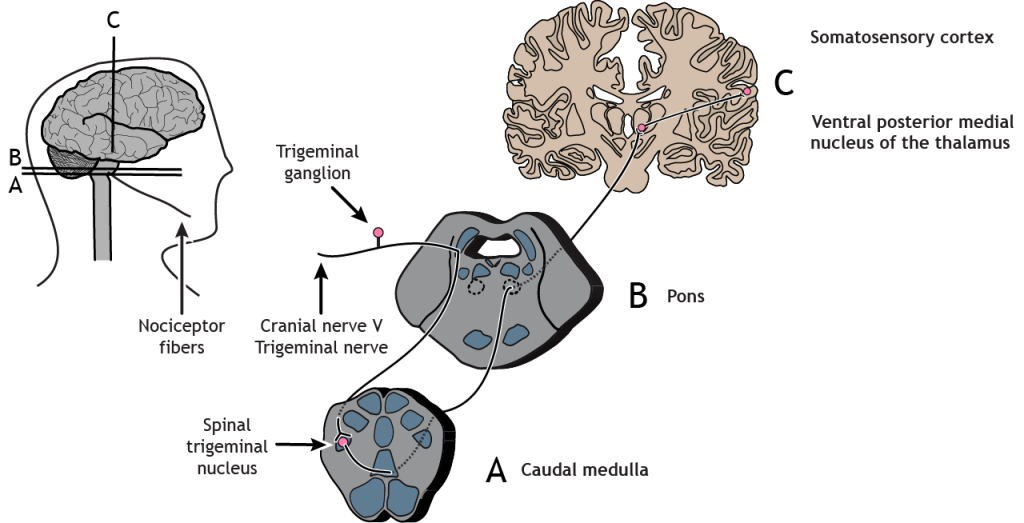
Nociceptors in the face and head send info to the brain primarily through cranial nerve V, the trigeminal nerve. The first-order neurons have their cell bodies in the trigeminal ganglion, located just outside of the brainstem. The fibers enter the brainstem and descend to the spinal trigeminal nucleus in the medulla, where they synapse on a second-order neuron. The second-order neurons cross the midline and project up to the ventral posterior medial nucleus of the thalamus. These neurons then send projections to the face region of the somatosensory cortex.
Sensitization
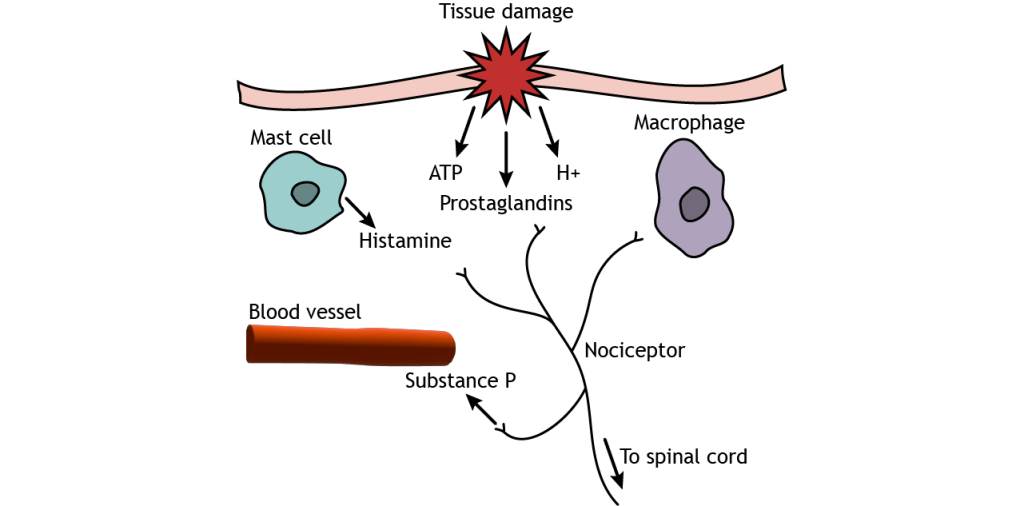
Pain sensitization occurs after injury and causes the feeling of pain in situations that would not normally cause pain. Tissue damage causes pain signals to be sent to the CNS, as seen above, but the injured tissue also releases substances like prostaglandins, neurotransmitters, substance P, cytokines, and protons, which cause inflammation and begin the healing process. Additionally, non-neuronal cell types such as mast cells and macrophages come to the injured site, releasing more inflammatory substances. These chemicals, particularly prostaglandins, however, can act on nociceptors and cause cellular changes that allow the receptors to be more sensitive to stimuli. This results in a decreased threshold for pain sensation. There are two types of pain sensitization. Hyperalgesia is increased pain in response to a stimulus that normally causes pain but less intensely, like having a second injury at or near a previously injured site. Allodynia is pain sensation in response to a stimulus that would normally not cause pain, like a light touch on a sunburn.
Modulation of Pain
Pain signals that enter the CNS can be modified.
Peripheral
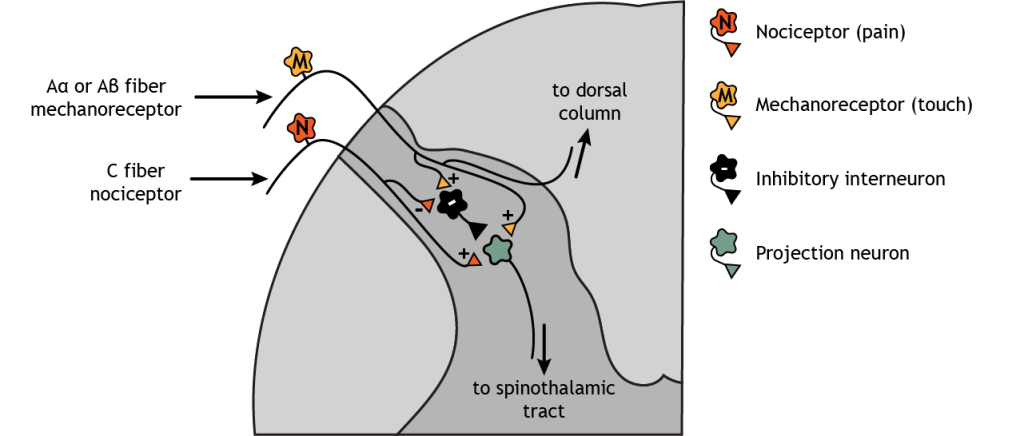
Think of a time when you cut a finger or stubbed a toe. It is common after an injury like this to put pressure at or near the injured location – squeeze the hurt finger or toe. This touch stimulation can decrease the pain sensation felt because sensory mechanoreceptors send signals through inhibitory interneurons in the dorsal horn of the spinal cord to pain neurons that ascend to the brain. This effect is known as the gate theory of pain.
One type of therapy that is believed to activate this process is transcutaneous electrical nerve stimulation (TENS) therapy. TENS units, which use small electrical impulses at the site of the pain, are often used to minimize both long- and short-term pain in joints and muscles.
Central
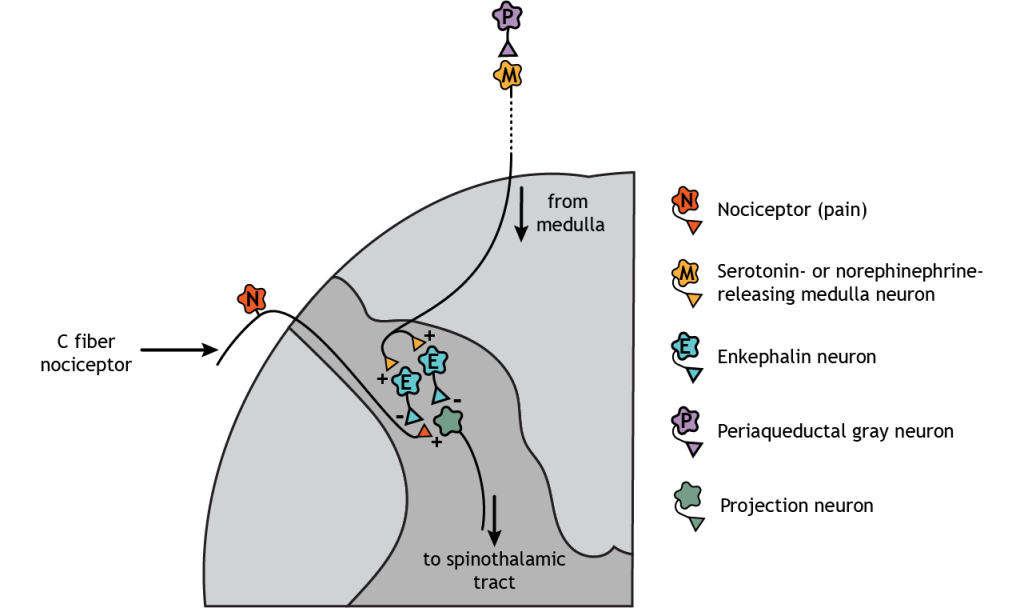
Descending regulation of pain also occurs. In this case, neurons from the medulla, which are innervated by the periaqueductal gray (PAG), descend and synapse in the dorsal horn of the spinal cord. The medulla neurons release either serotonin or norepinephrine onto enkephalin-releasing interneurons. These interneurons inhibit both the nociceptors and the second-order pain neurons that project to the brain. External stimulation of the PAG results in widespread analgesia.
Key Takeaways
- There are multiple types of nociceptors in the skin that are activated by different types of damaging stimuli
- Nociceptors express specialized proteins that convert noxious stimuli into electrical potentials during sensory transduction
- Nociceptor afferents synapse in the dorsal horn of the spinal cord. Information immediately decussates and synapses in the ventral posterior nucleus of the thalamus
- Information from the neck and body synapse in the ventral posterior lateral nucleus of the thalamus
- Information from the head and face synapse in the ventral posterior medial nucleus of the thalamus
- Pain sensitization occurs after injury and causes the feeling of pain in situations that would not normally cause pain
- Pain signals can be modified via peripheral and central nervous system processes
Test Yourself!

