46 Neurodegenerative Diseases: Motor
Neurodegenerative diseases cause neurons to lose either their function or structure over time, potentially leading to cell death in either the central or peripheral nervous system. These diseases can be devastating because there are no known ways to reverse the neurodegeneration. There are multiple diseases that are classified as neurodegenerative diseases and affect millions of people worldwide. We will focus on the two most prevalent neurodegenerative diseases: The motor disease Parkinson’s disease that affects the basal ganglia, and Alzheimer’s disease.
Parkinson’s Disease
Symptoms and Risk Factors
Parkinson’s disease is a neurodegenerative movement disorder that causes bradykinesia (slowness of movement), akinesia (difficulty initiating movement), a resting tremor, muscle rigidity, and changes to posture and locomotion. Although most symptoms are motor, there are mild cognitive and psychiatric changes, such as apathy, anhedonia, mood disturbances, or depression.
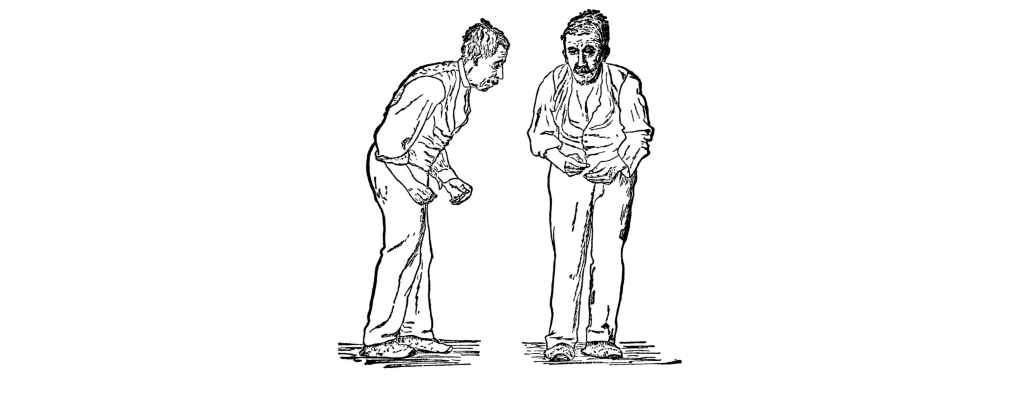
Advanced age is the primary risk factor, as an estimated 2% of people over the age of 60 develop Parkinson’s disease. It is also shows a sex difference with men being diagnosed more than women. Other environmental factors contribute to risk, such as repeated traumatic brain injury (suspected in Muhammad Ali) or occupational exposure to heavy metals, insecticides, or other neurotoxins. A small percentage of cases are early onset (21 – 50 years old; Michael J. Fox was diagnosed at 30), and have a strong genetic component. The disease causes significant decreases in life expectancy and quality of life.
Basal Ganglia Dysfunction
A loss of dopaminergic neurons within the substantia nigra (a midbrain structure) contributes to basal ganglia circuitry disruption in Parkinson’s disease. This loss of substantia nigra cells can be visualized postmortem without the use of histology due to the natural black pigmentation of the substantia nigra cells.
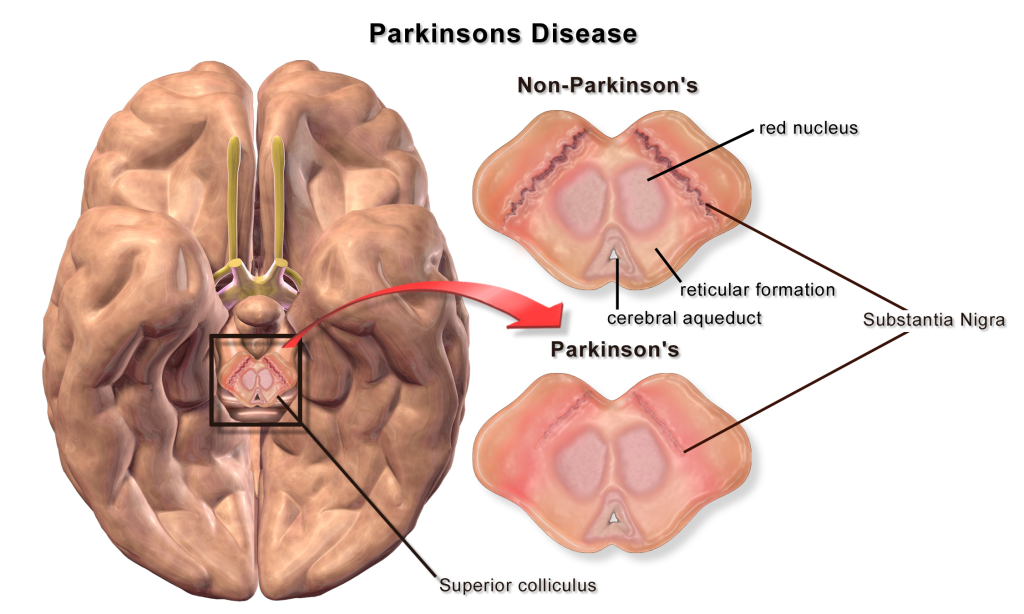
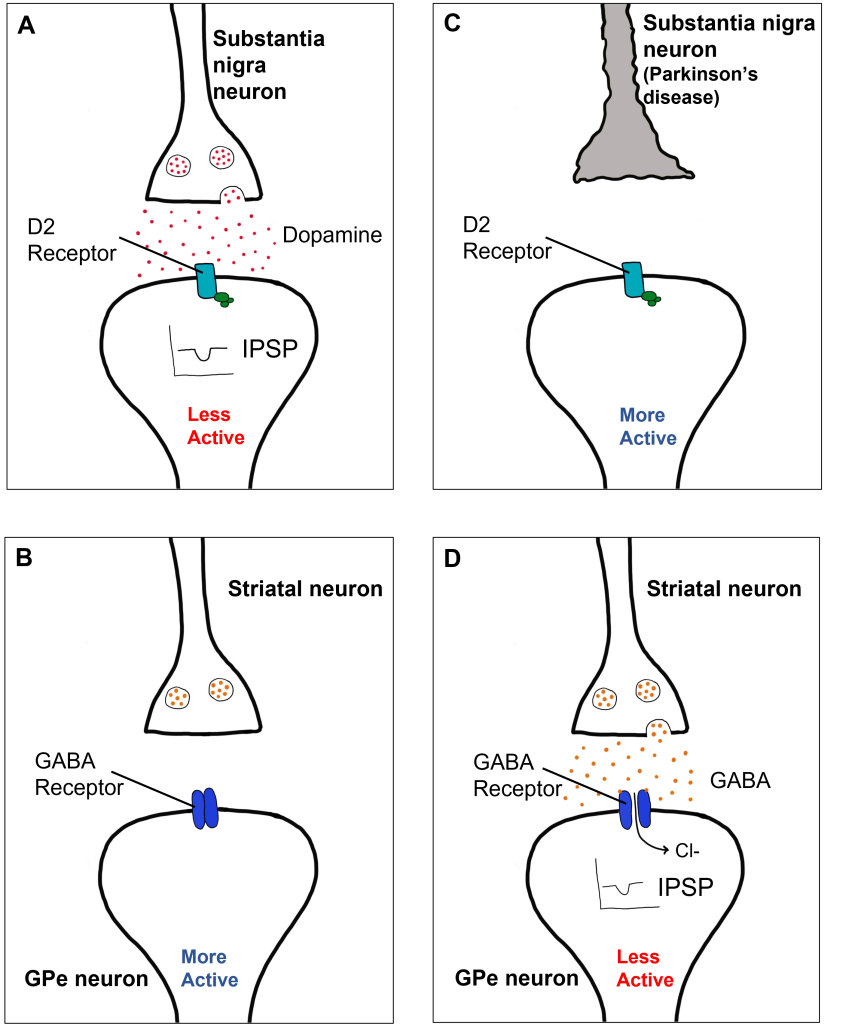
When the substantia nigra cells die, they are no longer able to release dopamine at the striatum appropriately. When less dopamine is released into the striatum, the two populations of striatal neurons are affected differently dependent on the type of dopamine receptor that they express. Recall the basal ganglia circuitry from the previous chapter.
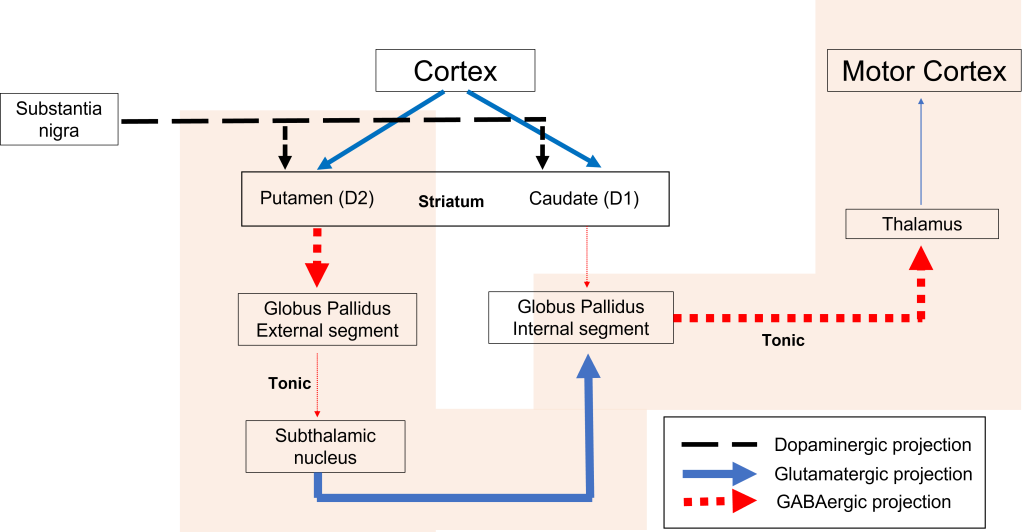
Direct Pathway Dysfunction
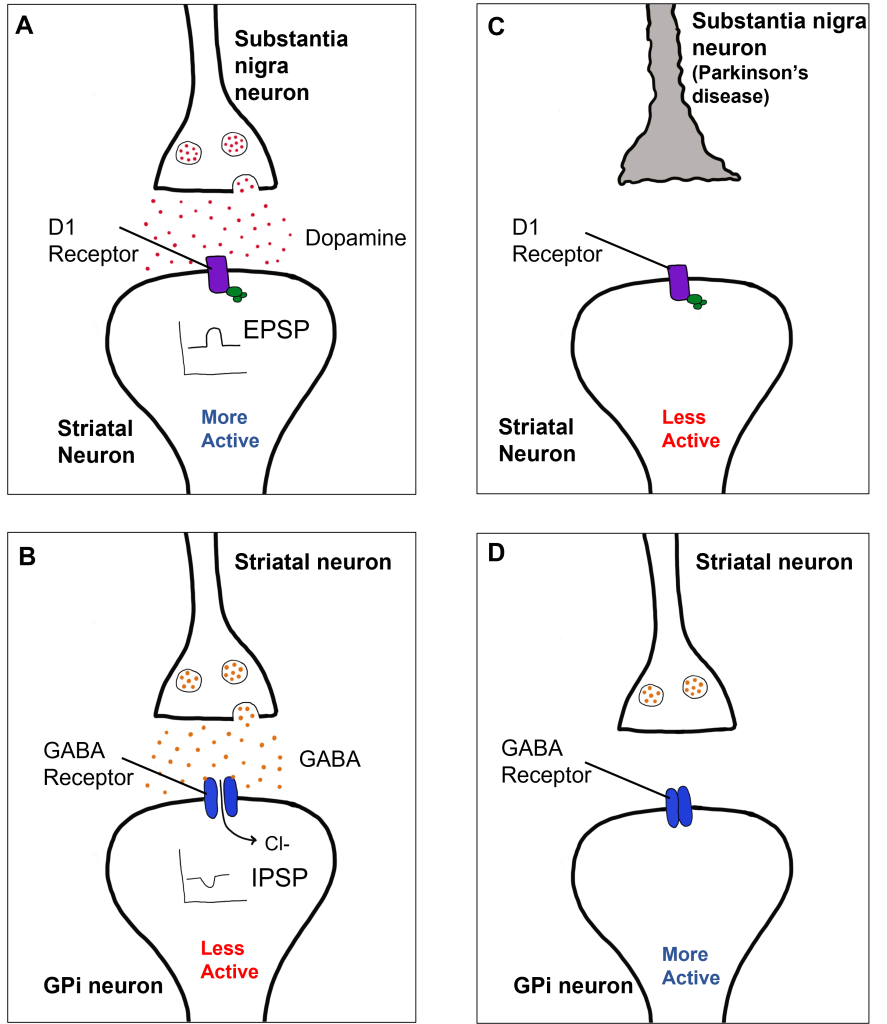
Within the direct pathway, striatal cells (within the caudate) express excitatory D1 receptors that project directly to the globus pallidus internal segment. In the direct pathway, typically, dopamine binds to D1 receptors on striatal neurons, increasing caudate activity. These striatal neurons release GABA onto the globus pallidus internal segment (GPi) neurons, causing the GPi neurons to be less active. When the Gpi neurons are less active, they release less GABA onto the thalamic neurons, increasing thalamic neuron activity and the excitatory projections to the motor cortex, thus increasing movement.
In Parkinson’s disease, there is decreased release of dopamine onto the striatal caudate neurons that express D1 receptors, making them less active. Now that the striatal neurons are not as active, they release less GABA onto GPi neurons, disinhibiting them. The GPi is now more active, releasing more GABA onto thalamic neurons. In response, the thalamic neurons are less active and send less excitatory messages to the cortex, thus causing a decrease in movement overall.
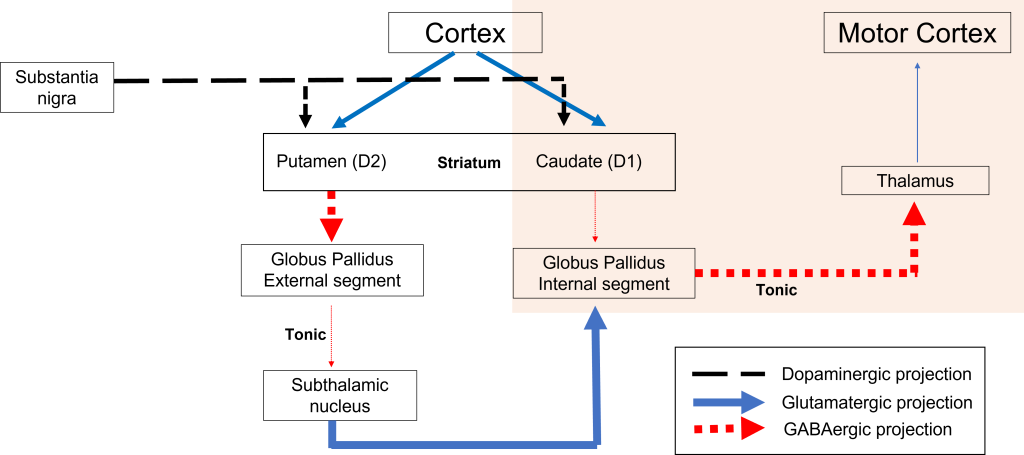
Indirect Pathway Dysfunction
In the indirect pathway, dopamine typically binds to inhibitory D2 receptors on striatal (putamen) neurons, inhibiting striatal neuron activity. These striatal neurons then release less GABA onto the globus pallidus external segment neurons (GPe), causing the GPe neurons to be more active. When the GPe neurons are more active, they release more GABA onto the subthalamic nucleus neurons, decreasing subthalamic neuron activity and the excitatory projections to the GPi. Decreasing activation of the GPi causes the GPi to release less GABA onto the thalamus, which then causes more activation of the motor cortex, thus increasing movement.
In Parkinson’s disease, there is decreased release of dopamine onto the striatal neurons that express D2 receptors, making them more active. Now that the striatal neurons are more active, they release more GABA onto GPe neurons. This inhibits the GPe neurons, causing them to release less GABA onto subthalamic nucleus neurons, disinhibiting the subthalamic nucleus. Increasing the excitatory inputs from the subthalamic nucleus onto the GPi will cause the GPi to release more GABA onto the thalamus. In response, the thalamic neurons are less active and send less excitatory messages to the cortex, thus causing a decrease in movement overall.
Diagnosis and Treatment
Parkinson’s disease is considered idiopathic because there is not a single known cause. Unfortunately, there are not measurable biomarkers (blood tests, brain scans) to diagnose Parkinson’s disease. Rather, diagnosis of individuals is typically done by a neurologist and a detailed exam. This can be further confirmed by giving medications that are used to treat Parkinson’s disease and determining if symptoms decrease.
A diagnosis of Parkinson’s disease is confirmed post-mortem through:
- The loss of dopaminergic substantia nigra cells.
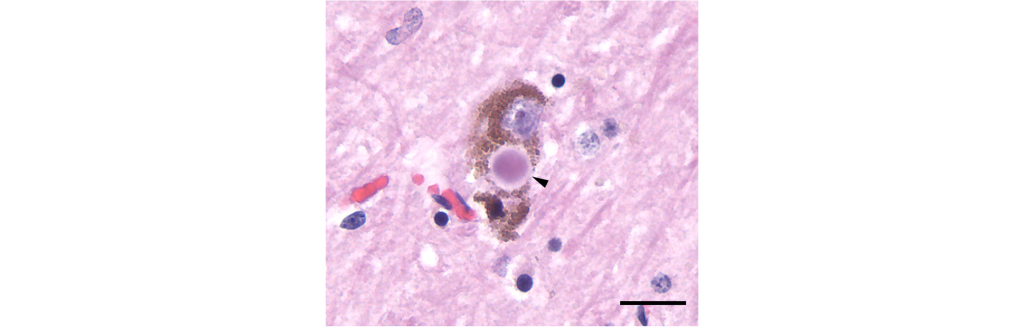
- Presence of Lewy bodies.
Lewy bodies are intracellular alpha-nuclein protein aggregates that are believed to potentially displace healthy neurons and lead to the neurodegeneration observed in the disease.
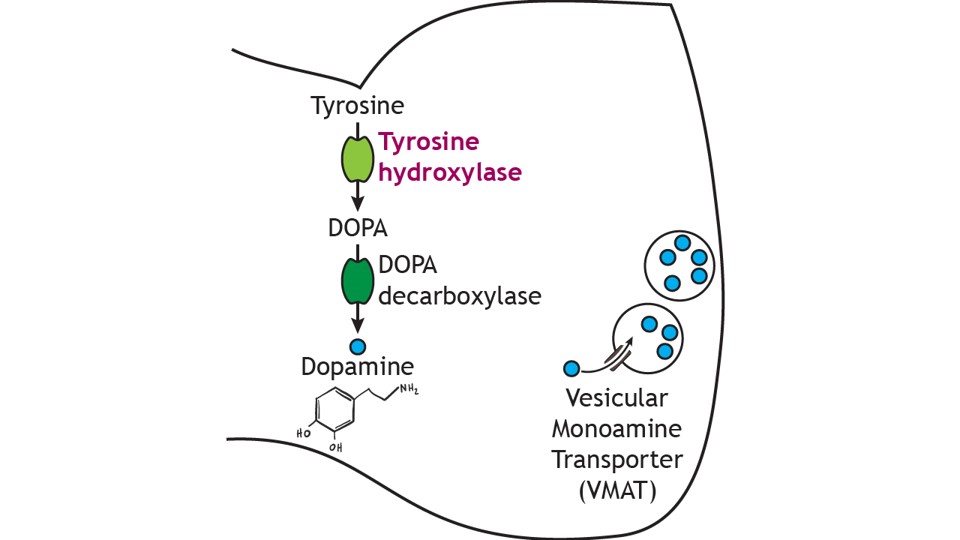
Dopamine cannot be administered as a treatment to those with Parkinson’s disease to replace the loss of dopamine from the substantia nigra due to the inability of dopamine to cross the blood-brain-barrier. Instead, the major drug treatment for the motor symptoms of Parkinson’s disease has been administration of the drug levodopa (L-DOPA). L-DOPA is an intermediate in the synthesis of dopamine. Typically, it is converted into dopamine through the activity of DOPA decarboxylase. It is typically co-administered with other drugs to help prolong its effects. Unfortunately, there are many side effects of L-DOPA including nausea, joint stiffness, dyskinesias (involuntary movements and tics), and psychosis.
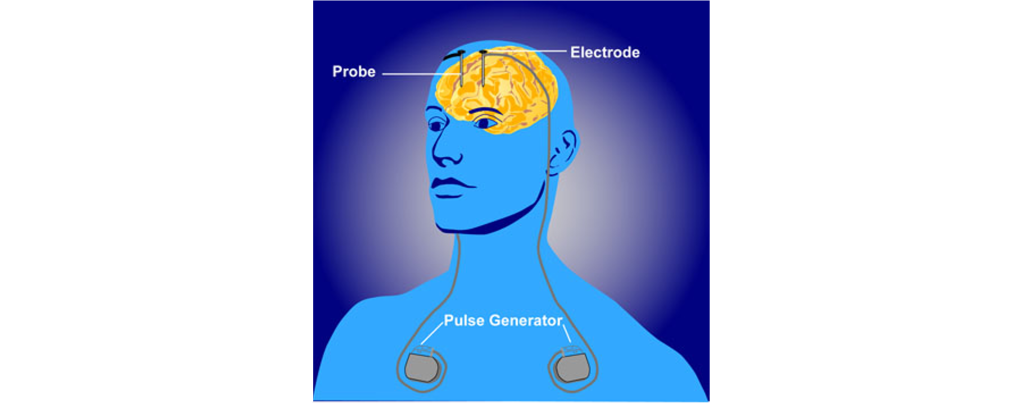
In a medical intervention called deep brain stimulation (DBS), a surgeon implants permanently indwelling electrodes directly into brain tissue. These electrodes are controlled by an external battery pack that delivers preprogrammed stimulation protocols. DBS in the STN is used to alleviate the symptoms of Parkinson’s disease.
Key Takeaways
- Parkinson’s disease is a neurodegenerative disease that causes dopaminergic substantia nigra cells to die, causing dysfunction in the basal ganglia circuitry.
- L-DOPA and deep brain stimulation are treatments for Parkinson’s disease.
Attributions
Portions of this chapter were remixed and revised from the following sources:
- Foundations of Neuroscience by Casey Henley. The original work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
- Open Neuroscience Initiative by Austin Lim. The original work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Media Attributions
- Parkinson’s disease Symptoms © Herter, Christian Archibald adapted by Valerie Hedges is licensed under a Public Domain license
- Parkinson’s disease substantia nigra © Bruce Blaus adapted by Valerie Hedges is licensed under a CC BY (Attribution) license
- Parkinson’s diseases effect on D1 receptor function © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Parkinson’s disease direct pathway Basal Ganglia model © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- D2 Receptor Effects
- Parkinson’s disease indirect pathway basal ganglia model © Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Lewy Body © Tulemo adapted by Valerie Hedges is licensed under a CC BY-SA (Attribution ShareAlike) license
- dopamine synthesis © Casey Henley adapted by Valerie Hedges is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Deep brain stimulation © NIMH adapted by Valerie Hedges is licensed under a Public Domain license
Neurological disorder of motor function resulting from the loss of dopamine-producing cells in the substantia nigra
Neurodegenerative disease that causes cell death and dementia
slowed motor movements
difficulty initiating movement
loss of pleasure
anatomical adaptation that restricts certain substances from being exchanged between the blood and brain tissue
involuntary movements

