Chapter 1: Acid–Base Reactions
 As we will see, organic reactions can be classified using a small set of reaction types—the largest and most all-encompassing of which are those involving acid–base reactions. Understanding acid–base reactions, therefore, provides a broadly useful conceptual framework within which to consider a wide range of organic reactions. Although it is likely that you have already been introduced to acid–base reactions (especially if you used the CLUE general chemistry curriculum[1]), we are going to review this class of reactions in order to emphasize their general features. Our goal is that you learn how to recognize their role in a range of reaction mechanisms; understanding how and why acid–base reactions occur will give you to a set of tools to understand phenomena as diverse as why most drugs are usually administered as in their salt form (a conjugate acid or base), why biological systems are buffered to specific pH levels (and why different pH levels are found in different cellular and organismic compartments), and why molecular oxygen (O2) transport systems require a metal ion complex (within the proteins involved, e.g. myoglobin, hemoglobin, cytochromes). As we will see, acid–base reactions are by far the most common types of reactions in biological systems.
As we will see, organic reactions can be classified using a small set of reaction types—the largest and most all-encompassing of which are those involving acid–base reactions. Understanding acid–base reactions, therefore, provides a broadly useful conceptual framework within which to consider a wide range of organic reactions. Although it is likely that you have already been introduced to acid–base reactions (especially if you used the CLUE general chemistry curriculum[1]), we are going to review this class of reactions in order to emphasize their general features. Our goal is that you learn how to recognize their role in a range of reaction mechanisms; understanding how and why acid–base reactions occur will give you to a set of tools to understand phenomena as diverse as why most drugs are usually administered as in their salt form (a conjugate acid or base), why biological systems are buffered to specific pH levels (and why different pH levels are found in different cellular and organismic compartments), and why molecular oxygen (O2) transport systems require a metal ion complex (within the proteins involved, e.g. myoglobin, hemoglobin, cytochromes). As we will see, acid–base reactions are by far the most common types of reactions in biological systems.
A quick review of the models of acid–base reactions.
There are a number of ways to discuss acid–base reactions, depending on what aspects of the reaction we want to highlight. They range from the extremely simplified (and not useful) Arrhenius model, to the Brønsted–Lowry model that we use only for reactions in which protons are transferred, and finally to the Lewis model, which can encompass any type of acid–base reaction.
Arrhenius: The Arrhenius acid–base model is probably the first acid–base model that you were introduced to in the course of your education. In this model, when an acid dissolves in water it dissociates to release a hydrogen ion (H+); when a base dissolves it releases a hydroxide ion (–OH).
Acid: HCl(g) + H2O [latex]\rightarrow[/latex] H+ (aq) + Cl– (aq) (sometimes written as or HCl(aq))
Base: NaOH(s) + H2O [latex]\rightarrow[/latex] Na+(aq) + –OH(aq)
Acid–Base Reaction: HCl (aq) + NaOH(aq) [latex]\rightarrow[/latex] NaCl(aq) + H2O (l)[2]
Although simple, the Arrhenius model is not particularly useful when it comes to understanding the reactions considered in organic chemistry. This of course raises the obvious question: so why are we mentioning it? The answer is two fold: i) because you might well vaguely remember it as a description of acid–base behaviors and ii) so that we can consider why it is not useful and why you should not use it. The Arrhenius acid–base model applies only when water is the solvent—as we will see many organic reactions do not occur in water. The Arrhenius model also, falsely implies that there are free protons (H+) roaming around in water and it restricts bases to those substances that release a hydroxide ion. Finally, it implies that an acid can exist independent of a base—and vice versa, which doesn't make a great deal of sense.
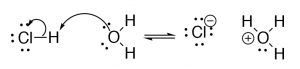
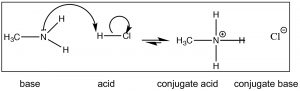
Brønsted–Lowry: The Brønsted–Lowry model is a much more useful and flexible model for considering acid base reactions. In this model an acid is a proton (H+) donor and a base is a proton acceptor. In the Brønsted–Lowry model you cannot have an acid without a base, and vice versa; the acid has to donate its H+ to something (the base), and similarly the base has to accept it. The H+ doesn't just “drop off"—it is transferred.[3] In the case of reactions that occur within aqueous solution, the H+ is transferred to a water molecule to form H3O+. Consider, as an example, HCl; in aqueous solution HCl transfer a H+ group to a water molecule. The products are H3O+ (the conjugate acid of water) and Cl–, the conjugate base of HCl.
|
HCl(g) + |
H2O(l) |
⇆ |
H3O+ (aq) |
+ |
Cl–(aq) |
|
acid |
base |
|
conjugate acid |
|
conjugate base |
|
HCl |
+ |
NH3 |
⇄ NH4+ + |
Cl– |
|
acid |
|
base |
conjugate acid |
conjugate base |
In the Brønsted–Lowry model, as for all chemical reactions considered at the molecular level, there is the possibility for the reaction to reverse, which is denoted by the use of equilibrium arrows (⇄).

from a source of electrons to a sink. Here the source is the lone pair on the oxygen, and the sink is the hydrogen (which has a δ+ due to its bonding to a Cl) The second arrow moves from the source (the bond between H and Cl, to the sink—the electronegative Cl which ends up with the negative charge, while the O that donated the original electron pair ends up with a positive charge).
The Lewis model encompasses the Brønsted–Lowry model, that is, all Brønsted–Lowry acid–base reactions that can be described using the Lewis model. However, the Lewis model extends the range of reaction types that can be considered as acid–base reactions. Take for example the reaction of ammonia (NH3) and boron trifluoride (BF3). This reaction is classified as a Lewis acid–base reaction, but it is not a Brønsted acid–base reaction.
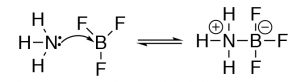
Why use different models of acid–base chemistry? While at first the idea of using different models to explain acid–base chemistry may be a little confusing. Why not use the all-encompassing Lewis model for everything? It turns out that both the Brønsted–Lowry and Lewis models are particularly useful depending on the system under consideration. The trick is to recognize which is the most useful when describing, predicting, and explaining a particular type of chemical reaction.[5]
In our explorations in organic chemistry we will be using both Brønsted–Lowry (proton transfer) and Lewis (electron pair donation) models to describe acid–base chemistry, depending on the type of reaction. In practice, the Brønsted–Lowry model is simple and useful; it tells you what is happening (proton transferred from acid to base) but nothing about the mechanism by which the H+ moves. For that we must turn to the Lewis model, which tells us how the electrons rearrange during the reaction. It is also important to keep in mind why these reactions happen—they are caused by an electrostatic interaction between two oppositely charged parts of molecules: δ– is attracted to δ+.
One further note, all reactions are initiated by random collisions of molecules, but only collisions that allow the electrostatic interaction of the acid and base to occur are productive (that is, collisions that involve two similarly charged parts of molecules will not give rise to a reaction. Again we will have more to say about this later.
Acid–Base Reaction Direction and Position of Equilibrium
Acid base reactions begin because of electrostatic interactions, but the extent to which the reaction proceeds depends on the relative Gibbs free energy of the reactants and products, that is, the overall Gibbs free energy change (ΔG) for the reaction. This is a subtle but important point: the reaction does not occur because the products are more stable, it occurs because there is an attractive force between two reactants that have polar structures, As we will see, we can predict the relative amounts of reactants and products in a mixture (at equilibrium), based both on an understanding of molecular structures and by comparing their pKa.
Acid Strength (using the Brønsted–Lowry model): The strength of an acid, that is the degree to which it donates H+ to (or accepts electron pairs from) other molecules, depends on a number of factors including, obviously, the strength of the base (that is the degree to which the base donates electron pairs to other molecules) it reacts with. Acid and base strengths are usually reported using water as the solvent (i.e. as the base or acid respectively), so that acid strengths can be compared directly. Since biological reactions take place in aqueous solution we will be able to extend our understanding of simple acid base reactions to much more complex ones as we move forward.
The reaction for any acid HA is:
HA + H2O ⇄ H3O+ + A–
We can estimate the extent of the reaction (i.e., how far the reaction goes, that is the concentrations of reactants and products when the reaction reaches equilibrium) by determining the equilibrium constant Ka.
Ka = [H3O+][ A–]/[ HA]
In contrast to strong inorganic acids (such as HCl, or HNO3), the equilibrium constants for many organic acids are small (ranging from 10–1 to 10-55) and it is more common to report pKa – which, as you will remember, is = –log Ka. A strong acid such as HCl has a large Ka (in fact it is so large as to be meaningless) and therefore a very small (negative) pKa.
Some representative Ka and pKa values.
|
Acid |
Ka |
pKa |
|
HCl (hydrochloric acid) |
~107 |
–7 |
|
CF3COOH (Trifluoroacetic acid) |
3.2 x 10–1 |
0.5 |
|
HF (hydrofluoric acid) |
7.2 x 10–4 |
3.14 |
|
CH3COOH (acetic acid) |
1.8 x 10–5 |
4.8 |
|
H20 |
10-14 |
14 |
|
CH3CH2OH (acetic acid) |
10-16 |
16 |
| NH4+ (ammonia in NH4Cl) | 5.6 x 10–10 | 9.25 |
| CH4 (methane) | ~10–55 | 55 |
It helps to be able to interpret these numbers in terms of the extent of the associated reaction. For example, water (which acts as both an acid and a base) dissociates to a very small extent. In a liter of pure water, which contains ~54 moles of water molecules (or ~54 x 6.02 x 1023 molecules or ~3.25 x 1025 molecules), ~10-7 moles (or ~10-7 x 54 x 6.02 x 1023 molecules or ~3.25 x 1016 H3O+ ions). The weaker the acid the higher the pKa (can you explain why that is the case and what it means in terms of the relative concentrations of species at equilibrium?).
Questions to answer:
- Explain why acids and bases are always (as pairs) found together in a system.
- What is meant by the terms conjugate acid or conjugate base?
- In the Lewis model for the HCl + water reaction, explain why you draw the arrow pointing from O to H.
- Complete these acid base reactions and predict the relative amounts of reactants and products when the reaction reaches equilibrium for each reaction. Explain your predictions using your knowledge of atomic and molecular structures and electronegativity.
CH3NH2 + HCL ⇄
CH3NH2 + H20 ⇄
CH3NH- + H20 ⇄
CH3NH3+ + H20 ⇄
Organic Acids and Bases
Having reviewed acids and bases using rather simple molecules (HCl and NH3), let us move on to the more complex world of organic acids and bases, how to identify them, how to determine relative strengths, and how to predict what will happen in any given mixture. We begin by comparing the pKa's of some organic acids. Let us begin with ethanol (pKa ~16), a molecule that we typically do not consider to be an acid, and acetic acid (pKa 4.8). There is clearly a huge difference between the pKa’s of these two molecules, the question is can we understand why this is the case?
If we draw out their structures we see that both have (as expected) the acidic hydrogen bonded to the electronegative oxygen. (Make sure you remember why the hydrogens bonded to carbons are not as acidic as those bonded to oxygen). So why the huge difference in pKa's? To answer this question we have to remember that the extent of the reaction depends on the relative thermodynamic stability of the products—that is, the system containing the conjugate base of the acid and the hydronium ion. The reactions and conjugate bases of the two are shown here (↓). 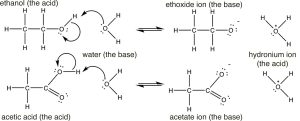 Based on their pKa values, we would predict that the ethanol dissociation reaction is rare (few ethoxide ions form) while the acetic acid dissociation reaction is more frequent. However note that even in the case of the acetic acid only about 3% of the acid molecules are dissociated in a 1M solution.
Based on their pKa values, we would predict that the ethanol dissociation reaction is rare (few ethoxide ions form) while the acetic acid dissociation reaction is more frequent. However note that even in the case of the acetic acid only about 3% of the acid molecules are dissociated in a 1M solution.
The first step in both reactions appears to be more or less the same, an electron pair from the oxygen in water forms a bond to the electron deficient hydrogen while the O–H bond of the acid breaks and the electrons originally associated with it the move back to the oxygen. The difference between the two reactions lies mainly in the way that the negatively charged conjugate bases (ethoxide and acetate) behave, and the way that they are solvated by the solvent (water). For ethoxide (ethanol’s conjugate base), the extra negative charge is localized onto the oxygen, which leads to a concentration of charge. Water molecules are strongly attracted to the ethoxide anion, an interaction that limits the mobility of the Resonance Structures Resonance Hybrid water molecules and results in a decrease in entropy (ΔS is negative). In contrast, in acetate (acetic acid’s conjugate base), the negative charge is delocalized onto both oxygens (even though it is often drawn as if it was associated with one but not the other). We can illustrate this in two ways (or more!) by drawing arrows to indicate how the extra electron pair can move from one oxygen to the other; it looks like this (→).
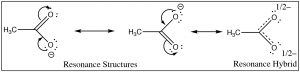 The actual structure has a partial negative charge on both oxygens. This pair of structures is often referred to as a resonance structure and the process is termed resonance but the name is misleading. In fact the actual structure, the resonance hybrid, does NOT involve the electrons (and the double bond) moving back and forth between the two oxygen atoms. By a biological (and not completely sensical) analogy we might say it is a mule or a hinny—the offspring of a cross between a horse and a donkey.[6] Just as a mule (or a hinny) is not bouncing back and forth between being a horse and being a donkey, so the resonance hybrid actually exists as a new species[7], with an actual structure that is partway between the two (drawn) resonance structures. In this case, we are using two bonding models (a valence bond and a delocalized molecular orbital model) to describe the structure of acetate anion. The localized valence bond model involves a sigma single bond framework that connects the atoms and provides the molecular shape. The delocalized molecular orbital model describes a pi bond that connects both Os to the C. We can visualize the anion as a planar sp2 hybridized carbon connected to a methyl group and two oxygens by sigma bonds together with a 3 atom two electron pi bond that extends over the O–C–O framework (→). The result is that in the acetate ion the negative charge is delocalized over two oxygens, rather than being concentrated on only one atom as it is in the
The actual structure has a partial negative charge on both oxygens. This pair of structures is often referred to as a resonance structure and the process is termed resonance but the name is misleading. In fact the actual structure, the resonance hybrid, does NOT involve the electrons (and the double bond) moving back and forth between the two oxygen atoms. By a biological (and not completely sensical) analogy we might say it is a mule or a hinny—the offspring of a cross between a horse and a donkey.[6] Just as a mule (or a hinny) is not bouncing back and forth between being a horse and being a donkey, so the resonance hybrid actually exists as a new species[7], with an actual structure that is partway between the two (drawn) resonance structures. In this case, we are using two bonding models (a valence bond and a delocalized molecular orbital model) to describe the structure of acetate anion. The localized valence bond model involves a sigma single bond framework that connects the atoms and provides the molecular shape. The delocalized molecular orbital model describes a pi bond that connects both Os to the C. We can visualize the anion as a planar sp2 hybridized carbon connected to a methyl group and two oxygens by sigma bonds together with a 3 atom two electron pi bond that extends over the O–C–O framework (→). The result is that in the acetate ion the negative charge is delocalized over two oxygens, rather than being concentrated on only one atom as it is in the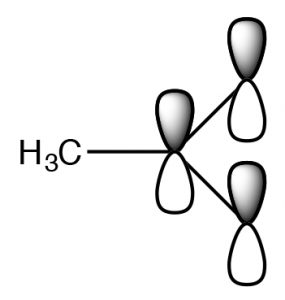 ethoxide ion. The result is that the interactions of the acetate with solvent water molecules is not as strong, so that the water molecules are not as ordered, meaning that the water is not as ordered around the molecule and the entropy change is not as negative. The effects of delocalizing charge over more than one atom play a major role in predicting the outcomes of a wide range of reactions. We note that ΔS is still negative since the creation of a charged species still leads to increased ordering of solvent molecules.
ethoxide ion. The result is that the interactions of the acetate with solvent water molecules is not as strong, so that the water molecules are not as ordered, meaning that the water is not as ordered around the molecule and the entropy change is not as negative. The effects of delocalizing charge over more than one atom play a major role in predicting the outcomes of a wide range of reactions. We note that ΔS is still negative since the creation of a charged species still leads to increased ordering of solvent molecules.
One way to predict whether charge can be delocalized is to determine whether resonance structures can be drawn for the charged species. For example: try convincing yourself that you cannot draw resonance structures for ethanol.
Resonance is not the only way to stabilize charge. Typically, resonance occurs through aconjugated pi bond system, such as occurs within the –CO2– part of an organic acid, but how do we account for the difference in the acidities of acetic acid (pKa 4.8) and trifluoroacetic acid (→) (pKa 0.5), even though they both have the carboxylate functional group? The difference between the two lies in the fact that the charge on the trifluoroacetate anion is delocalized by two distinct mechanisms. As in acetate, the negative charge is delocalized by resonance 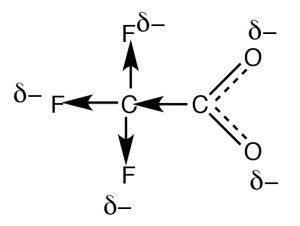 through the pi bonding system; in addition it is also delocalized onto the fluorines by the fact that the highly electronegative fluorine atoms (more electronegative than O) withdraw electrons from the methyl carbon through the sigma bonds, which in turn withdraws electrons from the next carbon, and in turn from the two oxygens (a process known as “induction”). The result is that the negative charge is “smeared out” over even more atoms, making the anion even less likely to cause a solvent molecule ordering (reducing the effect on ΔS). As you might expect, the inductive effect is distance dependent (perhaps you can predict the effect of adding more CH2 groups between the CF3 and CO2 groups).
through the pi bonding system; in addition it is also delocalized onto the fluorines by the fact that the highly electronegative fluorine atoms (more electronegative than O) withdraw electrons from the methyl carbon through the sigma bonds, which in turn withdraws electrons from the next carbon, and in turn from the two oxygens (a process known as “induction”). The result is that the negative charge is “smeared out” over even more atoms, making the anion even less likely to cause a solvent molecule ordering (reducing the effect on ΔS). As you might expect, the inductive effect is distance dependent (perhaps you can predict the effect of adding more CH2 groups between the CF3 and CO2 groups).
Questions to answer:
- Using resonance structures predict which is more acidic: C6H5OH or CH3CH2OH?
- Draw structures to show how sodium ethoxide and sodium acetate are solvated in water, and use them to show why the negative entropy change for the formation of sodium acetate is smaller than that of sodium ethoxide.
- Consider the pKa's of the three chlorobutanoic acids: CH3CH2CHClCOOH (pKa 2.86), CH3CHClCH2COOH (pKa 4.05), and CH2ClCH2CH2COOH (pKa 4.53). Draw structures and use them to explain why these carboxylic acids have different pKa's.
Organic Bases
As noted previously, there are no acids without bases, and vice versa. Even if we are only discussing H+ (proton) transfer, it is (arguably) easier to think about the base using a Lewis model. That is, a base has an electron pair available for donation into a bond with the acid. Recall that almost everything that has a pair of non-bonding electrons (sometimes called a lone pair) can act as a base. The most common types of organic bases often have a nitrogen atom somewhere in their structure. If we compare the basicity of N, O and F, each of which have lone pairs that are could potentially be donated, nitrogen is the least electronegative and therefore the best able to donate its electrons into a bond, since its lone pair is least attracted by the nucleus. Fluorine, the most electronegative element, holds its electrons very close to the nucleus, and under normal circumstances would not be considered as a base.
Oxygen, since it is more electronegative than nitrogen is not as strong a base, therefore when ammonia and water are mixed, the only reaction that occurs (and that to a relatively small extent) is a proton transfer from water to ammonia.
NH3 + H2O ⇆ NH4+ + –OH
The equilibrium constant for this reaction is 1.8 x 10–5 (most of the species in the mixture at equilibrium are reactants).
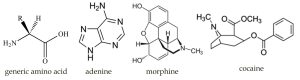 Here are some organic bases (→). Note that they are components of a wide range of biologically active molecules, including DNA, hormones and pharmaceuticals. As we will see the basic nitrogen provides an important way to understand the reactivity of a particular species.
Here are some organic bases (→). Note that they are components of a wide range of biologically active molecules, including DNA, hormones and pharmaceuticals. As we will see the basic nitrogen provides an important way to understand the reactivity of a particular species.
 For now, however, let us start with a simpler base such as methylamine (CH3NH2) the simplest nitrogenous organic base. Methylamine reacts with acids (↓) in much the same way that ammonia does; it will react with a strong acid like HCl(aq) to produce methylammonium chloride.
For now, however, let us start with a simpler base such as methylamine (CH3NH2) the simplest nitrogenous organic base. Methylamine reacts with acids (↓) in much the same way that ammonia does; it will react with a strong acid like HCl(aq) to produce methylammonium chloride.
Recall that the position of equilibrium can be predicted by comparing the strength (pKa’s) of the two acids. HCl (pKa –7) is a much stronger acid than CH3NH3+ (pKa ~10) and therefore we predict that the equilibrium of the methylamine + HCl reaction will lie well to the right. Now consider the reaction in which methylamine reacts with acetic acid (↓).
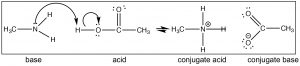
Again we can predict the position of equilibrium by comparing pKa’s of the conjugate acids (acetic acid 4.8 and CH3NH3+ ~ 10). Notice that you can predict the structure of the products simply by following the flow of electrons. We could change the CH3 (methyl) groups on either methylamine and acetic acid to a wide range of different groups and still be able to predict the product easily, as long as you recognize that the reaction that takes place is a (simple) proton transfer (acid–base). For example, look at the structure of cocaine (above): can you predict what will happen if it were reacted with acetic acid? What would be the structure of the product?
Molecules that contain both an acid and a base:
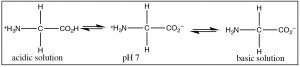
The most common example of a molecule that act as both an acid and a base is of course water because it has both a potentially acidic hydroged, and a lone pair that can accept the proton. However, since this is organic chemistry, where water is not as common a solvent, let us consider the class of molecules that have both acidic and basic domains simultaneously. The most biologically important such molecules are the amino acids, which have both an amino group and a carboxylic acid. A subset of the possible amino acids are those used in biological systems to assemble polypeptides. Amino acids (or rather the α-amino acids) contain both a carboxylic acid and an amino group attached to a central carbon (the α-carbon). The generic structure is given here (→) where R stands for a wide range of side chains.[8] 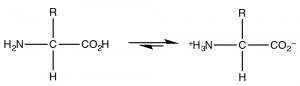 At pH 7 the amino acid exists in what is know as a zwitterionic form, in which the carboxylic acid group is negatively charged while the amino group is positively charged. At no time would an amino acid (dissolved in water) exist in an un-ionized form. We can predict what form would be present at different pH’s by considering the pKa's of the species involved.
At pH 7 the amino acid exists in what is know as a zwitterionic form, in which the carboxylic acid group is negatively charged while the amino group is positively charged. At no time would an amino acid (dissolved in water) exist in an un-ionized form. We can predict what form would be present at different pH’s by considering the pKa's of the species involved.
Effect of pH on acid base reactions
So far, we have discussed situations when the acid or base is added to solution of pure water. Pure water has a pH of 7, and [H3O+] = [–OH]. Now let us consider what happens when we change the pH of the solution. For example consider a situation in which we dissolve a simple organic acid (CH3CO2H, acetic acid) in a solution that has a pH > 7, that is where the [–OH] > [H3O+]; under these conditions the extent of the acetic acid’s ionization is increased. 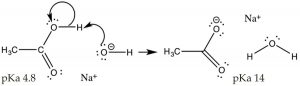 Recall that in 1M acetic acid only ~3% of the acid is ionized at pH 7. If we change the solution to make it basic by adding NaOH, the excess of strong base (–OH) will completely deprotonate the acid. At equilibrium, the reaction will now favor products over reactants (i.e. it will move to the right). What we have done here is drive the acetic acid ⇆ acetate reaction to the right, increasing the concentration of acetate, which is an application of Le Châtelier’s principle). Note that Na+, derived from the addition of the NaOH used to adjust the pH, is present but does not take part in the reaction – for this reason it referred to as a “spectator ion”. Another, perhaps simpler, way to predict the outcome of this reaction is to use the pKa values of the two acids (CH3CO2H, 4.8 and H2O, 14), clearly acetic acid is a much stronger acid than water, and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. What we have done here is change the acetic acid, which is a polar organic molecule, into acetate, an ionic species.
Recall that in 1M acetic acid only ~3% of the acid is ionized at pH 7. If we change the solution to make it basic by adding NaOH, the excess of strong base (–OH) will completely deprotonate the acid. At equilibrium, the reaction will now favor products over reactants (i.e. it will move to the right). What we have done here is drive the acetic acid ⇆ acetate reaction to the right, increasing the concentration of acetate, which is an application of Le Châtelier’s principle). Note that Na+, derived from the addition of the NaOH used to adjust the pH, is present but does not take part in the reaction – for this reason it referred to as a “spectator ion”. Another, perhaps simpler, way to predict the outcome of this reaction is to use the pKa values of the two acids (CH3CO2H, 4.8 and H2O, 14), clearly acetic acid is a much stronger acid than water, and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. What we have done here is change the acetic acid, which is a polar organic molecule, into acetate, an ionic species.
Acetic acid is a small organic molecule; since it is polar it can interact with water (though intermolecular forces), therefore acetic acid is very soluble in water (indeed it is miscible with water (it has unlimited solubility.)[9] But now, let us consider the effect of increasing the length of the hydrocarbon group of the organic acid on its molecular properties. Acetic acid has a methyl (CH3–) group, the smallest possible hydrocarbon. In contrast dodecanoic (lauric) acid has a 12-carbon hydrocarbon chain (CH3[CH2]11–) and has a solubility in water of 0.063 g/L (~30 mM) at 25 °C, which is much less that of acetic acid.[10] As the hydrocarbon (non-polar) part of the molecule increases in length, solubility in water decreases: the ΔG of the process of dissolving the organic acid in water becomes more positive. This decrease in solubility is primarily due to a negative entropy change (ΔS) caused by the self-organization of water molecules around the hydrocarbon “tail” of the molecule.  Now let us consider the behavior of ionized sodium dodecanoate (the sodium salt of dodecanoic acid); it, like many ionic species, it is soluble in water. Although as you may remember, this is a different form solubility – the soluble species is not isolated molecules but rather molecular complexes known as micelles (→).[11] The upshot of this is we can “solubilize” organic acids in water by deprotonating them, but if we then lower the pH, the organic acid will separate from solution again.
Now let us consider the behavior of ionized sodium dodecanoate (the sodium salt of dodecanoic acid); it, like many ionic species, it is soluble in water. Although as you may remember, this is a different form solubility – the soluble species is not isolated molecules but rather molecular complexes known as micelles (→).[11] The upshot of this is we can “solubilize” organic acids in water by deprotonating them, but if we then lower the pH, the organic acid will separate from solution again.
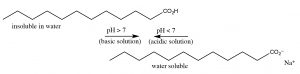
Organic bases can be solubilized in a similar way, except that now the solution must be made acidic. For example, a nitrogenous base with a large non-polar group such as dodecylamine (C12H27N) has a solubility of about 3.5 g/L (~20mM), but at acidic pHs it is completely soluble. Contrast the solubility of dodecyl amine with cadaverine (NH2CH2CH2CH2CH2CH2NH2), the compound that smells like its name, which is completely miscible with water because it has two polar amino groups. It turns out that we can predict the pH at which a particular acid or base protonates or deprotonates. You may recall from general chemistry that the pH of weak acids and their conjugate bases (like most organic species) can be described using the Henderson Hasselbalch equation (→).
One way to work with this equation is to note that [acid] = [conjugate pH = pKa + log [latex]\frac{[base]}{[acid]}[/latex] base] when the pH of the system is equal to pKa of the acid. At pH’s below pKa, [acid] > [conjugate base], there is more acid than base, and vice versa for pH > pKa. Therefore, by adjusting the pH we can change the concentrations of conjugate acid and base to suit our purposes, or we can predict the relative concentrations at any pH. For example, acetic acid with a pKa of 4.8 would have 50% CH3CO2H, and 50% CH3CO2–Na+, at a pH of 4.8. If the pH falls below 4.8 the concentration of protonated acid will increase, and if it rises the concentration of acetate ion will increase.
This ability to transform an organic substance from an insoluble (in water) molecule to a soluble ionic species can be very useful. One common example stems from the fact that many pharmaceutical drugs are organic substances that are insoluble in aqueous solutions (like cytoplasm or blood). If these substances were introduced into the body in their non-ionized form they would not dissolve, and therefore be inactive. If you check the labels on many prescription bottles you will see that the drug is administered as a salt. Consider norepinephrine (→), a hormone that is often administered intravenously to counteract the effects of allergic reactions. 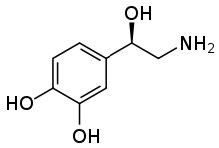 It is administered as a salt of tartaric acid to ensure that it is soluble in the blood stream.
It is administered as a salt of tartaric acid to ensure that it is soluble in the blood stream.
You may come across another example of this phenomenon (that acids are soluble in basic solution, and bases are soluble in acid solution) if you take the organic chemistry laboratory course. If your product has an acidic or basic moiety in its structure, you can extract the substance into aqueous (acid or basic) solution, washing away all the organic by-products with an organic solvent, and then regenerating the acidic or basic substance. This is an important purification method for many substances, because it allows the compound of interest to be separated into aqueous solution and then regenerated simply by adding or subtracting a proton.
 When we consider biomolecules (that is, organic molecules found in organisms) the situation is not so clear cut; most biomolecules have a variety of acidic and basic groups as part of their structure. Even the simplest amino acid, glycine (→) exist in a variety of protonated and deprotonated forms depending on the pH.
When we consider biomolecules (that is, organic molecules found in organisms) the situation is not so clear cut; most biomolecules have a variety of acidic and basic groups as part of their structure. Even the simplest amino acid, glycine (→) exist in a variety of protonated and deprotonated forms depending on the pH.
One thing that becomes clear is that individual amino acids are always charged regardless of the pH, so they are water-soluble. But the extent of the protonation/deprotonation reactions is pH dependent. As we will see this has a number of ramifications for a wide range of biological molecules, because they will behave very differently in different pH solutions. This is one important reason why most biological systems are buffered so that they remain at a fairly constant pH.
Questions to Answer:
- If you have a mixture of benzoic acid C6H5CO2H (pKa 4.2), toluene, C6H5CH3 and aniline hydrochloride (pKa of C6H5NH3+ 4.6). Which substance will be soluble in aqueous acidic solution, which will be soluble in aqueous basic solution, which will not be soluble in water?
- Outline a scheme for separating these three substances by using their differing solubilities in organic and aqueous solutions of different pHs.
Lewis Acids and Bases, Electrophiles and Nucleophiles
As we have seen, any reaction in which a proton (H+) is transferred from one molecule to another can be considered as a Lewis acid–base reaction, but now it is time to broaden the scope of Lewis acid–base reactions. The structural requirement for a Lewis base is essentially the same as those we discussed for a Brønsted base. That is, a Lewis base must have an accessible lone pair of electrons that can be donated into a bond with a Lewis acid. 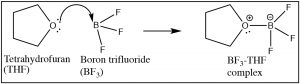 For example, many (but not all) nitrogen and oxygen containing molecules have such available lone electron pairs and so can be considered as Lewis bases.[12] It is the Lewis acid that can take a number of different forms (and so, can be harder to recognize). A Lewis acid must be able to accept a pair of electrons. In practice this means a variety of substances (besides H+) can act as Lewis acids: for example, any species with empty orbitals that are energetically accessible can be a Lewis acid.
For example, many (but not all) nitrogen and oxygen containing molecules have such available lone electron pairs and so can be considered as Lewis bases.[12] It is the Lewis acid that can take a number of different forms (and so, can be harder to recognize). A Lewis acid must be able to accept a pair of electrons. In practice this means a variety of substances (besides H+) can act as Lewis acids: for example, any species with empty orbitals that are energetically accessible can be a Lewis acid. 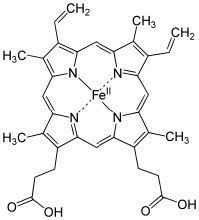 Common examples of this situation are compounds of Group III elements (specifically B and Al); these have only three valence electrons. Examples include BF3 (↑) and AlCl3,[13] both of which have a partial positive charge on the central atom and an empty orbital that can accept electrons. Other examples of Lewis acids are metal ions such as Fe2+, Fe3+, Cu2+, and Mg2+; these, by definition, have empty orbitals. The same situation holds true for many transition metal salts, for example TiCl4 and NiCl2.[14] In biological systems, examples of Lewis acid–base complexes include the active site of the oxygen transport complex in hemoglobin (and myoglobin), which consists of an iron ion complexed with 4 nitrogens, which are part of a porphyrin ring. A similar iron-porphyrin complex is found associated with the cytochrome proteins that participate in the ATP synthesis reaction associated with in the electron transport chain of the mitochondria (→). Chlorophyll, the green pigment that is part of the light capture system in algae and plants has a similar structure, except that the Lewis acid at the center of the complex is Mg2+ rather than Fe2+. This has the interesting effect of making chlorophyll species appear to be green, rather than the red observed in blood. This is caused by the difference energy gaps between the molecular orbitals in an Fe complex as compared to a Mg complex with a porphyrin ring. We will discuss this effect in more detail later. As we will also see later, Lewis acids are important class of reagents in organic chemistry because they can interact with a wide range of bases.
Common examples of this situation are compounds of Group III elements (specifically B and Al); these have only three valence electrons. Examples include BF3 (↑) and AlCl3,[13] both of which have a partial positive charge on the central atom and an empty orbital that can accept electrons. Other examples of Lewis acids are metal ions such as Fe2+, Fe3+, Cu2+, and Mg2+; these, by definition, have empty orbitals. The same situation holds true for many transition metal salts, for example TiCl4 and NiCl2.[14] In biological systems, examples of Lewis acid–base complexes include the active site of the oxygen transport complex in hemoglobin (and myoglobin), which consists of an iron ion complexed with 4 nitrogens, which are part of a porphyrin ring. A similar iron-porphyrin complex is found associated with the cytochrome proteins that participate in the ATP synthesis reaction associated with in the electron transport chain of the mitochondria (→). Chlorophyll, the green pigment that is part of the light capture system in algae and plants has a similar structure, except that the Lewis acid at the center of the complex is Mg2+ rather than Fe2+. This has the interesting effect of making chlorophyll species appear to be green, rather than the red observed in blood. This is caused by the difference energy gaps between the molecular orbitals in an Fe complex as compared to a Mg complex with a porphyrin ring. We will discuss this effect in more detail later. As we will also see later, Lewis acids are important class of reagents in organic chemistry because they can interact with a wide range of bases.
Electrophiles and Nucleophiles
The next logical step in expanding our ideas about Lewis acids and bases is to consider reactions that involve carbon. We will first consider reactions in which carbon acts like the Lewis acid, that is, it accepts a pair of electrons to form a new bond with a Lewis base. So, what situations would we make a carbon act in this way? We can rule out (for now) carbon compounds with an empty orbital (akin to boron). Why? Because all stable carbon compounds form 4 bonds and there are no low-lying empty orbitals that can be used to accept electrons.
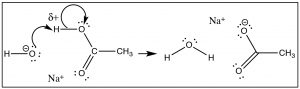 But let us first look at the proton (H+) transfer reaction as a model (→). In this case the bond with the Lewis base (OH-) is formed at the same time as the bond to the conjugate base (of the acid) is broken. We see that the δ+ on the H means that the bond to the H is partially ionized. The H is “on the way” to becoming H+—a species that does have an empty and accessible orbital. The δ+ on the H attracts the negative (or δ–) charge on the base, and the reaction is initiated, forming a new bond between the O and the H, and at the same time breaking the old O–H bond.
But let us first look at the proton (H+) transfer reaction as a model (→). In this case the bond with the Lewis base (OH-) is formed at the same time as the bond to the conjugate base (of the acid) is broken. We see that the δ+ on the H means that the bond to the H is partially ionized. The H is “on the way” to becoming H+—a species that does have an empty and accessible orbital. The δ+ on the H attracts the negative (or δ–) charge on the base, and the reaction is initiated, forming a new bond between the O and the H, and at the same time breaking the old O–H bond.
We can imagine that a carbon compound with a δ+ on the C might behave in a very similar way. 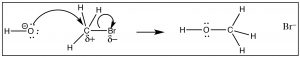 In this molecule (H3CBr) the C-Br bond is polarized so that there is a small positive charge on the C, which attracts the negatively charged hydroxide (→). Formation of the O–C bond occurs with the simultaneous breaking of the C-Br bond.
In this molecule (H3CBr) the C-Br bond is polarized so that there is a small positive charge on the C, which attracts the negatively charged hydroxide (→). Formation of the O–C bond occurs with the simultaneous breaking of the C-Br bond.
Consider the analogies between these two reactions – the mechanisms of how and why the electrons move are similar. The only real difference between the two reactions is that in the first an H+ is transferred from an O (on the carboxylic acid) to the OH–, while in the second, a methyl group is transferred to the OH–. Now for a change in nomenclature: when such a reaction involves a C atom (a carbon center) rather than call the electron deficient carbon a Lewis acid, we call it an electrophile (electron or negative charge loving). Similarly, the hydroxide ion (which acts as a Lewis base) is now called a nucleophile (positive charge loving). This change in terminology is not just to confuse students! In fact, there are subtle differences between Lewis acids and bases and electrophiles and nucleophiles that make the distinction between the two useful. In particular, while all Lewis bases are nucleophiles, as we will see, not all nucleophiles are bases.
So now we have to ask ourselves, what factors make a particular C within a molecule an electrophile? How can we recognize a nucleophile? What criteria do we use to estimate the strength of a particular electrophile or an nucleophile? Can we ever get carbon to act as a nucleophile? If we can answer these questions, we can predict the outcome of a wide array of reactions.
What makes a particular carbon an electrophile?
The simplest of organic compounds are hydrocarbons, and the simplest of hydrocarbons are known as alkanes. Alkanes typically have the formula CnH2n+2 (or if CnH2n if there is one ring of carbons, subtract 2H for every extra ring). All of the bonds within an alkane are sigma (single) bonds; they do not contain pi (double) bonds.[15] In an alkane, each carbon is fully saturated, it makes four single bonds and (as noted above) there are no double or triple bonds. C-C bonds are of course, completely non-polar since the electrons are equally distributed between two identical atoms, however C-H bonds are also relatively non-polar since the electronegativities of C and H are quite similar. In practice this means that alkanes are limited in their reactivity. The most common reactions that an alkane can take part in are reactions with oxygen to produce CO2 and H2O. This reaction is highly exothermic, although there is a significant activation energy, so it requires an initial input of energy (typically a spark, a burning match) to start the reaction, but then the energy from the formation of the strong C=O and O-H bonds (which is why the reaction is exothermic) can be used to initiate more reaction. The actual reaction mechanism is complex; it proceeds via a series of highly reactive (unstable) free radicals (species with unpaired electrons)[16]. While this reaction is obviously highly important—this is still how we generate much of the energy to run our cars and electrical power stations, from an organic chemistry perspective it is not very interesting in large part because it is more or less uncontrollable. That is, if you have enough oxygen once started the reaction generates CO2 and H2O, regardless of which hydrocarbon you begin with. [17]
All this is to say that alkanes are not good candidates for the kinds of reactions we are considering, they have neither nucleophile nor electrophilic carbons. So, let us turn our attention to carbon compounds with elements other than C and H and both sigma and pi bonds (this is, of course, the rest of organic chemistry). Here we find a very different situation: the range of reactions and the types of products can seem almost unlimited. While it is impossible (and certainly undesirable) to memorize every reaction and every potential product, it is possible to organize your understanding of chemical systems so that you can make plausible predictions as to which reactions may occur. By knowing reaction mechanisms, and when they are relevant, you can also predict which reactions will occur and therefore what products will form. As you might recognize, this is the same strategy we have used to consider acid–base reactions, which can be understood much more broadly than simple proton (H+) transfer reactions. Thinking in an electrophile-nucleophile context provides an entrée into much of organic chemistry.
For reactions (other than reactions involving free radicals, like combustion) to occur, there is generally a “handle” within the substrate: a place where the electron density is not evenly distributed, a site at which reactants of opposite charge interact (and react). In the example we used previously, the electrophilic carbon has a δ+ on it; this partial charge arose because the C was bonded to a more electronegative element. Such a partially positively charged C is attractive to any species with a negative (or partial negative) charge. 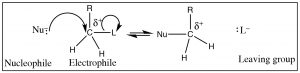 Note that, for now, we are going to restrict the type of carbon atom that we are considering to either a primary (that is a carbon with only one alkyl group (denoted by R) and 2 hydrogens, CH2R–) or a methyl carbon (CH3–). As we will see things become more complicated when we start to add more alkyl groups around the site of attack—so we will come back to that later.
Note that, for now, we are going to restrict the type of carbon atom that we are considering to either a primary (that is a carbon with only one alkyl group (denoted by R) and 2 hydrogens, CH2R–) or a methyl carbon (CH3–). As we will see things become more complicated when we start to add more alkyl groups around the site of attack—so we will come back to that later.
To identify such a partially positively charged C one would look for C’s bonded to groups (atoms) that are more electronegative, that is, that will act to withdraw electrons from the carbon (denoted by L below). But since carbon cannot form more than four bonds as the nucleophile comes in and forms a bond, another bond must break. The electronegative atom (L) (or group of atoms), is known as the “leaving group” (oh, how dull) needs to be stable when it leaves with the extra pair of electrons. We can, in fact, predict the characteristics of a good leaving group. For example, the bond to the leaving group should be polarized, and since the leaving group takes the electron pair with it, the group should be stable with this extra pair of electrons on it (L–). Another way of saying this is that the leaving group should be electronegative and breaking the C-L bond should produce a weak base. Halide ions are examples of good leaving groups, and their order of reactivity is I– > Br– > Cl– > F–. This ranking mirrors their acid strength rankings—that is, HI is the strongest acid and HF is the weakest—which means F– is the strongest base (and therefore least likely to leave)
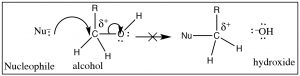 So, what about oxygen, in the form of an alcohol O–H group, as a leaving group? (→) It certainly fulfills the requirement that the C–O bond be polarized, but if you follow the reaction through it would mean that the leaving group would be a hydroxide ion (– OH), a very strong base. Therefore, alcohols (ROH) are not likely to be attacked by a nucleophile.
So, what about oxygen, in the form of an alcohol O–H group, as a leaving group? (→) It certainly fulfills the requirement that the C–O bond be polarized, but if you follow the reaction through it would mean that the leaving group would be a hydroxide ion (– OH), a very strong base. Therefore, alcohols (ROH) are not likely to be attacked by a nucleophile.
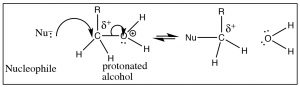 There are ways, however, ways to make an alcohol reactive. For example, if we can carry out the reaction in an acidic solution, the alcohol will be protonated (at least some of the time), and therefore the leaving group will be a water molecule, a stable entity (→).
There are ways, however, ways to make an alcohol reactive. For example, if we can carry out the reaction in an acidic solution, the alcohol will be protonated (at least some of the time), and therefore the leaving group will be a water molecule, a stable entity (→).
What makes a good nucleophile?
As we have noted, a Lewis base is also a nucleophile, so the trends you have learned about the strengths of Lewis bases also hold for nucleophiles. So, for example, nucleophilicity decreases across a row in the periodic table so NH3 > H2O > HF in the same way as base strength does (recall this is because the lone pair is more available on the N than on F). But since this is organic chemistry, we should have some organic groups dangling off the nucleophiles. 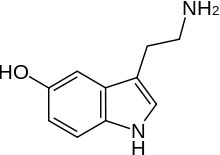 So for example, instead of a hydroxide nucleophile, we could use an alkoxide nucleophile (for example, CH3CH2O– Na+ sodium ethoxide), or we could use amine nucleophiles like serotonin (the nitrogen in the NH2 group here is more nucleophilic than the OH group, and the N in the ring). In addition, if we compare nucleophiles with the same nucleophilic atom, a negatively charged species is more nucleophilic than the uncharged form, so OH– > H2O, and NH2– > NH3 (and by analogy any organic derivatives behave the same way).
So for example, instead of a hydroxide nucleophile, we could use an alkoxide nucleophile (for example, CH3CH2O– Na+ sodium ethoxide), or we could use amine nucleophiles like serotonin (the nitrogen in the NH2 group here is more nucleophilic than the OH group, and the N in the ring). In addition, if we compare nucleophiles with the same nucleophilic atom, a negatively charged species is more nucleophilic than the uncharged form, so OH– > H2O, and NH2– > NH3 (and by analogy any organic derivatives behave the same way).
Besides the nucleophiles that are easily recognizable because they are bases, there is another class of nucleophiles that are somewhat different; they have a lone pair of electrons, but they are not particularly basic. The most common examples are the halide ions, which are weak bases and good leaving groups. So, the question arises: why are halide ions such good nucleophiles? The reason for this has to do with their polarizability (that is, the extent to which an electron cloud can get distorted) of the nucleophile. A very large anion-like iodide has a very polarizable electron cloud because the electrons extend much further out from the nucleus than, for example, the electron cloud in fluoride. This means that the electron cloud for iodide can begin partial bond formation to the carbon much earlier than the one for fluoride, and therefore iodide reacts much faster than fluoride.[18] This logic allows us to explain why the nucleophilicity of halide ions increases as you go down a group: I– > Br– > Cl– > F–.
Although we will return to this reaction in greater detail later, let us take a look at the range of possible reactions that this generic scheme enables us to predict – with the caveat that we are considering simple carbon substrates. Reactions like this are called nucleophilic substitutions, because the species that attacks the carbon is a nucleophile, and the overall effect of the reaction is that we have substituted the nucleophile for the leaving group. This particular example is called an SN2 reaction which stands for Substitution, Nucleophilic, 2nd Order, and we will come back to discuss the reaction in much more detail later.
Another type of carbon nucleophile
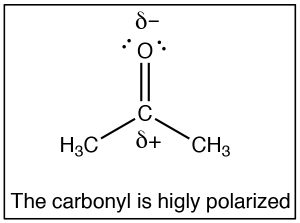 The SN2 reaction is a mainstay of organic chemistry, by varying the substrate (carbon electrophile) the leaving group, and the nucleophile we can construct a huge array of different compounds. Another very important type of compound that has an electrophilic carbon (i.e. a carbon that is subject to nucleophilic attack) is one which contains a carbonyl group (C=O). The carbonyl group is highly polarized, with a large δ+ on the carbon. This can be rationalized by the idea that there are two bonds to the electronegative oxygen and therefore the oxygen has even more tendency to pull electrons away from the carbon than a single bonded oxygen would.
The SN2 reaction is a mainstay of organic chemistry, by varying the substrate (carbon electrophile) the leaving group, and the nucleophile we can construct a huge array of different compounds. Another very important type of compound that has an electrophilic carbon (i.e. a carbon that is subject to nucleophilic attack) is one which contains a carbonyl group (C=O). The carbonyl group is highly polarized, with a large δ+ on the carbon. This can be rationalized by the idea that there are two bonds to the electronegative oxygen and therefore the oxygen has even more tendency to pull electrons away from the carbon than a single bonded oxygen would. 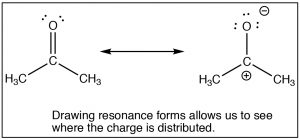 One way to visualize this is to draw resonance structures for the carbonyl group as shown, where the electrons from the double bond are now located on the O. We will come back to how to draw resonance forms in much more detail later.
One way to visualize this is to draw resonance structures for the carbonyl group as shown, where the electrons from the double bond are now located on the O. We will come back to how to draw resonance forms in much more detail later.
Once we understand how compounds with carbonyl groups are polarized, we can predict (at least for the first step) how these compounds will react. For example, if we have a reasonably good nucleophile (here shown as Nu–)we might predict that it would attack at the carbonyl carbon. 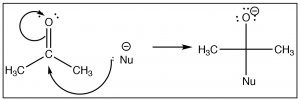 The difference in this reaction and an SN2 reaction is that there is no leaving group. Instead the electrons from one of the C-O bonds move onto the oxygen as shown.
The difference in this reaction and an SN2 reaction is that there is no leaving group. Instead the electrons from one of the C-O bonds move onto the oxygen as shown.
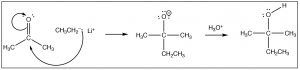
There are a number of ways that this reaction can continue, the most obvious of which is that if the reaction is in contact with a solvent that has acidic protons (e.g. water or an alcohol), the O– can simply protonate in an acid base reaction. As we will see later, the course of the reaction also depends on what the nucleophile is. Here we will give the simplest example which is the reaction of a ketone (acetone) with a carbon nucleophile (CH3CH2Li, ethyl lithium). For now, we will not worry about how to make ethyl lithium, but rest assured it is possible! When the negatively charged carbon electrophile adds to the carbonyl we make a new carbon-carbon bond. This is followed by addition of water to protonate the oxygen, to produce an alcohol. The overall reaction is a nucleophilic addition.
Questions to Answer
Try your hand at predicting the outcomes for these reactions by drawing arrow pushing mechanisms.
- CH3CH2I + NaOH →
- CH3Br + NaN3 →
- CH3CH2Cl + NH2CH3 →
- CH3OH + H+ →
What nucleophile and electrophile would you react together to form these products?
- CH3OH + Br–
- CH3CH3NH3+ + Cl–
- Construct a generalizable model for the SN2 reaction and explain the role of the substrate (the carbon electrophile), the leaving group, and the nucleophile.
Construct a generalizable model for the nucleophilic addition reaction and explain the role of the substrate (the carbon electrophile), and the nucleophile. What functional groups would undergo a nucleophilic addition? - What would make a carbon in a compound a nucleophile? How could you go about making a particular carbon nucleophilic?
- If you did not take the CLUE general chemistry curriculum, we recommend that you take a look at the materials on the web here. ↵
- Here the nomenclature can be confusing, since when in solution HCl, NaCl, and NaOH exist primarily in their dissociated forms, i.e. H+ and Cl–, Na+ and Cl–, and Na+ and OH–. ↵
- In fact, later on we will use abbreviated notation to indicate the transfer of H+ from one molecule to another simply because there are so many reactions in which multiple proton transfers take place. However, you should always be aware that protons are always transferred (not simply ionized or dropped off). ↵
- We will have MUCH more to say about arrow pushing as we proceed, but it is good to get a head start on this skill. ↵
- The situation is similar to when we use different atomic and molecular structure models depending on our purpose. The most sophisticated of these models are based on quantum mechanical calculations, but in many cases, producing a calculated model (which considers each and every atom in a system) may be impossible or not even particularly useful; when a simpler model is adequate it makes sense to use it. As an example, when considering bonding within molecules we typically use the valence bond (VB) model in which we think of each bond as composed of two electrons that are attracted to both of the atomic nuclei involved in the bond. Alternatively we could use molecular orbital (MO) model, in which we consider bonding (and anti-bonding) orbitals that involve the entire molecule. Each MO contains a maximum of two electrons and the bonding interactions are considered over the whole molecule. While useful in certain contexts, the MO model is usually unnecessarily complex for everyday use. As we will see shortly, there are situations when we will choose to use one or another, or both models of bonding, depending on the circumstances. ↵
- Whether a mule or a hinny is produced from such a cross depends upon whether the horse in the mother or father and is due primarily to parental imprinting of genes expression within the placenta: http://www.ncbi.nlm.nih.gov/pubmed/23754418 ↵
- Please excuse this analogy, since hinnys and mules are typically sterile and so do not represent species: https://en.wikipedia.org/wiki/Mule#Fertility ↵
- There are 20 naturally occurring amino acids that are encoded for by the genetic code (actually there are 22 but selenocysteine and pyrrolysine are used only in a very few microbes). ↵
- The implication is that we can take pure acetic acid (known as glacial acetic acid) which is 17.4 Moles/L and add any amount of water and have a solution of acetic acid in water (or perhaps water in acetic acid, depending on the relative amounts of two compounds). ↵
- For fuller explanation of this phenomenon, you should review the chapter in CLUE on solubility (chapter 6). ↵
- In a micelle, the non-polar groups are oriented towards the center of the structure while the ionic “heads” are in contact with the water solvent. ↵
- Nitrogenous compounds with non-available lone pairs include ammonium salts (where the N is bonded to four other atoms), and aromatic heterocyclic compounds, where the lone pair is part of the aromatic pi system. ↵
- In fact AlCl3(l) exists as a dimers Al2Cl6, but this structure decomposes and then reacts as AlCl3 in the presence of a Lewis base. Diborane B2H6 is also a dimer of BH3, that will break apart to react as BH3. ↵
- In general transition metal complexes and ions have empty d orbitals that are energetically accessible. ↵
- If there are pi bonds present the substance is not an alkane – we will get to that presently ↵
- There is an excellent (but simple) video on the mechanism of reactions that produce flames such as this one. https://vimeo.com/40271657 ↵
- In fact there is a great deal of interest in controlling this reaction—that is, finding ways to take hydrocarbons and selectively introducing a functional group—rather than fully oxidizing the hydrocarbon. http://pubs.acs.org/doi/full/10.1021/acscentsci.6b00139 ↵
- Another very important factor in understanding nucleophile strength is the structure of the solvent. We will return to this idea later. ↵

