Fluid Statics
75 What Is a Fluid?
Learning Objectives
- State the common phases of matter.
- Explain the physical characteristics of solids, liquids, and gases.
- Describe the arrangement of atoms in solids, liquids, and gases.
Matter most commonly exists as a solid, liquid, or gas; these are the three common . Solids have a definite shape and volume. Liquids maintain a definite volume but take the shape of their container. Gases have neither definite shape nor volume and expand to fill their container. (Figure 75.1)
Liquids and gases are classified as because they yield to , unlike solids which resist such deformation. The extent to which fluids flow in response to these forces depends on a property called , which we’ll explore further in Viscosity and Laminar Flow; Poiseuille’s Law.
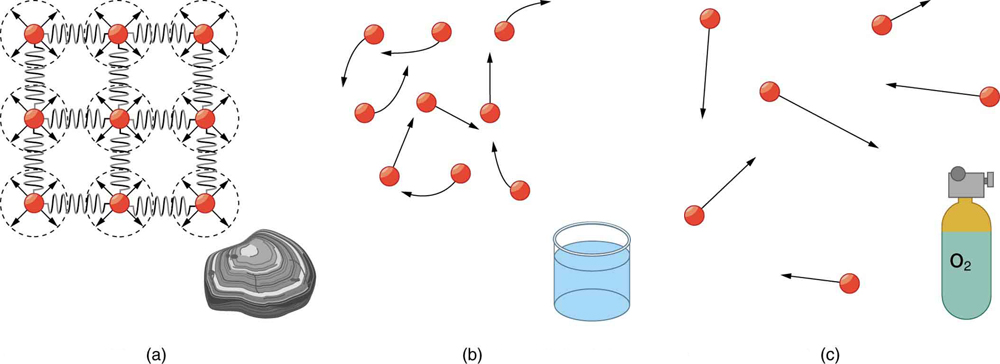
In solids, atoms are tightly packed in a regular structure. They vibrate but cannot easily move past one another. The forces between atoms act like stiff springs, resisting compression and shearing forces. This rigidity explains why solids maintain their shape and volume.
Connections: Submicroscopic Explanation of Solids and Liquids
The atomic and molecular structures of solids and liquids explain their macroscopic behaviors. This approach—linking submicroscopic structure to physical behavior—is a recurring theme in this course and is explored further in Conservation of Momentum.
By contrast, in liquids, atoms are still in close contact but are free to slide past one another. Liquids resist compression like solids, but they do not resist shearing forces and thus can flow. Liquids stay in an open container due to interatomic attraction, unless the container leaks.
In gases, atoms are spaced far apart and interact weakly. They move freely and rapidly, colliding with one another and the container walls. Because of the large spacing, gases are easily compressed and will escape from an open container. Both liquids and gases are considered fluids, but gases are distinct in being highly compressible.
Section Summary
- A is a substance that flows and yields to shearing forces. Both liquids and gases are fluids.
- Solids resist deformation because their atoms are fixed in a lattice structure and can only vibrate.
- Liquids flow and take the shape of their container, though they resist compression due to close atomic spacing.
- Gases flow and are easily compressed due to large spacing between atoms and weak intermolecular forces.
Conceptual Questions
- What physical characteristic distinguishes a fluid from a solid?
- Which of the following substances are fluids at room temperature: air, mercury, water, glass?
- Why are gases easier to compress than liquids and solids?
- How do gases differ from liquids?
Glossary
- fluids
- liquids and gases; a fluid is a state of matter that yields to shearing forces
liquids and gases; a fluid is a state of matter that yields to shearing forces

