Heat and Heat Transfer Methods
102 Phase Change and Latent Heat
Learning Objectives
- Examine the role of heat transfer in phase changes.
- Calculate energy required for melting, freezing, vaporization, or condensation.
In previous sections, we explored how heat transfer can change the temperature of a substance. However, not all heat transfer results in temperature change. When a substance undergoes a phase changexf—for example, when ice melts or water boils—its temperature remains constant during the transition. Consider the case of icicles melting from a sun-warmed roof or water freezing into ice in a cold freezer. These are everyday examples of heat transfer that cause a change in phase rather than temperature.
To melt a solid, energy must be supplied to break the molecular bonds that hold the material together in its ordered structure. This energy does not increase the substance’s temperature but instead disrupts the intermolecular forces. Likewise, additional energy is needed to vaporize a liquid as molecules must overcome even stronger cohesive forces to enter the gas phase. The temperature remains constant during these processes until the phase change is complete. Conversely, during freezing or condensation, energy is released as molecular bonds reform. This process is illustrated in Figure 102.2.

The amount of heat required to change the phase of a sample depends on the mass and the strength of intermolecular interactions. This is described by the following equations:
Here, [latex]Q[/latex] is the heat added or removed, [latex]m[/latex] is the mass of the substance, and [latex]L_{\text{f}}[/latex] and [latex]L_{\text{v}}[/latex] are the latent heat of fusion and vaporization, respectively. These constants vary by substance and are typically determined through experiment. Representative values are listed in Table 102.1.
Because phase changes involve breaking or forming molecular bonds, the energy involved is large. For example, to melt 1 kg of ice at [latex]0^\circ\text{C}[/latex], the required energy is:
This is the same amount of energy needed to raise 1 kg of liquid water from [latex]0^\circ\text{C}[/latex] to [latex]79.8^\circ\text{C}[/latex]—showing how energy-intensive phase changes can be. Vaporizing water requires even more energy: [latex]2256\ \text{kJ}[/latex] to convert 1 kg of water at [latex]100^\circ\text{C}[/latex] into steam at the same temperature (see Figure 102.3).

| Lf | Lv | |||||
|---|---|---|---|---|---|---|
| Substance | Melting point (ºC) | kJ/kg | kcal/kg | Boiling point (°C) | kJ/kg | kcal/kg |
| Helium | −269.7 | 5.23 | 1.25 | −268.9 | 20.9 | 4.99 |
| Hydrogen | −259.3 | 58.6 | 14.0 | −252.9 | 452 | 108 |
| Nitrogen | −210.0 | 25.5 | 6.09 | −195.8 | 201 | 48.0 |
| Oxygen | −218.8 | 13.8 | 3.30 | −183.0 | 213 | 50.9 |
| Ethanol | −114 | 104 | 24.9 | 78.3 | 854 | 204 |
| Ammonia | −75 | 108 | −33.4 | 1370 | 327 | |
| Mercury | −38.9 | 11.8 | 2.82 | 357 | 272 | 65.0 |
| Water | 0.00 | 334 | 79.8 | 100.0 | 22562 | 5393 |
| Sulfur | 119 | 38.1 | 9.10 | 444.6 | 326 | 77.9 |
| Lead | 327 | 24.5 | 5.85 | 1750 | 871 | 208 |
| Antimony | 631 | 165 | 39.4 | 1440 | 561 | 134 |
| Aluminum | 660 | 380 | 90 | 2450 | 11400 | 2720 |
| Silver | 961 | 88.3 | 21.1 | 2193 | 2336 | 558 |
| Gold | 1063 | 64.5 | 15.4 | 2660 | 1578 | 377 |
| Copper | 1083 | 134 | 32.0 | 2595 | 5069 | 1211 |
| Uranium | 1133 | 84 | 20 | 3900 | 1900 | 454 |
| Tungsten | 3410 | 184 | 44 | 5900 | 4810 | 1150 |
Phase changes also explain temperature regulation in the environment and the human body. In humid climates, temperatures rarely exceed [latex]35^\circ\text{C}[/latex] because energy from the sun goes into evaporating water, which stabilizes temperature. Likewise, the release of energy during condensation (e.g., morning dew) prevents temperatures from falling below the dew point.
The biological relevance of this is clear: for example, when we sweat, the evaporation of perspiration removes heat from the skin. This requires energy—about [latex]2428\ \text{kJ/kg}[/latex] at body temperature, which is more than at the boiling point. If the humidity is high, evaporation slows, and cooling is less efficient, which can elevate body temperature.
Calculate Final Temperature from Phase Change: Cooling Soda with Ice Cubes
Three ice cubes are used to chill a soda at [latex]\text{20º}\text{C}[/latex] with mass [latex]{m}_{\mathrm{soda}}=\text{0.25 }\text{kg}[/latex]. The ice is at [latex]0º\text{C}[/latex] and each ice cube has a mass of 6.0 g. Assume that the soda is kept in a foam container so that heat loss can be ignored. Assume the soda has the same heat capacity as water. Find the final temperature when all ice has melted.
Strategy
The ice cubes are at the melting temperature of [latex]0º\text{C}[/latex]. Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: first the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the temperature of this water rises. Melting yields water at [latex]0º\text{C}[/latex], so more heat is transferred from the soda to this water until the water plus soda system reaches thermal equilibrium,
The heat transferred to the ice is [latex]{Q}_{\text{ice}}={m}_{\text{ice}}{L}_{\text{f}}+{m}_{\text{ice}}{c}_{\text{W}}\left({T}_{\text{f}}-0º\text{C}\right)[/latex]. The heat given off by the soda is [latex]{Q}_{\text{soda}}={m}_{\text{soda}}{c}_{\text{W}}\left({T}_{\text{f}}-\text{20º}\text{C}\right)[/latex]. Since no heat is lost, [latex]{Q}_{\text{ice}}=-{Q}_{\text{soda}}[/latex], so that
Bring all terms involving [latex]{T}_{\text{f}}[/latex] on the left-hand-side and all other terms on the right-hand-side. Solve for the unknown quantity [latex]{T}_{\text{f}}[/latex]:
Solution
- Identify the known quantities. The mass of ice is [latex]{m}_{\text{ice}}=3\text{×}6.0 \text{g}=0\text{.}\text{018}\text{ kg}[/latex] and the mass of soda is [latex]{m}_{\text{soda}}=0\text{.}\text{25 }\text{kg}[/latex].
- Calculate the terms in the numerator:
[latex]{m}_{\text{soda}}{c}_{W}\left(\text{20º}\text{C}\right)=\left(0\text{.25 kg}\right)\left(\text{4186 J/kg}\cdot º\text{C}\right)\left(\text{20º}\text{C}\right)=\text{20,930 J}[/latex]
and
[latex]{m}_{\text{ice}}{L}_{\text{f}}=\left(0\text{.}\text{018 }\text{kg}\right)\left(\text{334,000}\text{ J/kg}\right)\text{=6012 }\text{J.}[/latex] - Calculate the denominator:
[latex]\left({m}_{\text{soda}}+{m}_{\text{ice}}\right){c}_{\text{W}}=\left(0\text{.}\text{25 }\text{kg + 0}\text{.}\text{018 kg}\right)\left(\text{4186 K/}\left(\text{kg}\cdot º\text{C}\right)\right)\text{=1122 J/º}\text{C}\text{.}[/latex]
- Calculate the final temperature:
[latex]{T}_{\text{f}}=\frac{\text{20},\text{930 J}-\text{6012 J}}{\text{1122 J/º}\text{C}}=\text{13º}\text{C.}[/latex]
Discussion
This example illustrates the enormous energies involved during a phase change. The mass of ice is about 7 percent the mass of water but leads to a noticeable change in the temperature of soda. Although we assumed that the ice was at the freezing temperature, this is incorrect: the typical temperature is [latex]-6º\text{C}[/latex]. However, this correction gives a final temperature that is essentially identical to the result we found. Can you explain why?
We’ve already learned that vaporization requires energy input: heat must be transferred into a liquid for it to become a gas. The reverse process, condensation, releases energy back to the surroundings. This may seem counterintuitive, since condensation often occurs on cold surfaces—like a glass of iced tea. However, for vapor molecules to condense, they must release energy. The energy released during condensation is exactly equal to the energy required to vaporize the same mass of liquid, and it can be calculated using:

Real-World Application
When water freezes, it also releases heat. This principle is used by Florida orange growers: when freezing temperatures threaten crops, they spray water on the trees. As the water freezes, it releases heat, which helps maintain the internal temperature of the fruit near [latex]0^\circ\text{C}[/latex], preventing cellular damage.

Another phase change of biological and environmental relevance is sublimation, the direct transition from solid to vapor. You may have observed snow vanishing without melting or noticed how dry ice (solid carbon dioxide) produces fog-like vapor. The reverse process, known as deposition, forms solids like frost directly from vapor, bypassing the liquid phase entirely.
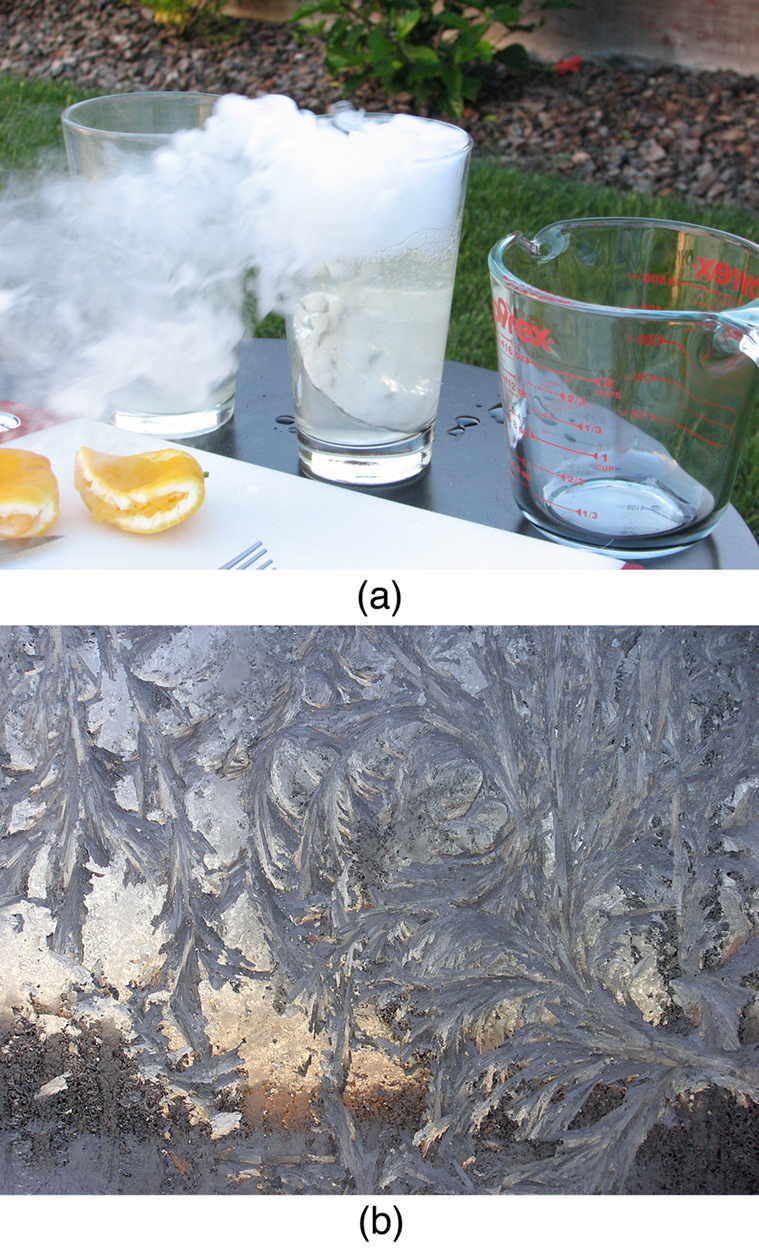
All phase transitions involve heat. For sublimation, the required energy is given by:
Here, [latex]L_{\text{s}}[/latex] is the latent heat of sublimation, the energy needed to convert 1 kg of solid directly into vapor. Like [latex]L_{\text{f}}[/latex] (for fusion) and [latex]L_{\text{v}}[/latex] (for vaporization), [latex]L_{\text{s}}[/latex] is specific to each material. Dry ice is commonly used as a refrigerant because it absorbs large amounts of heat as it sublimates. On the flip side, when water vapor undergoes deposition and forms frost, it releases energy to the environment.
Problem-Solving Strategies for Phase Change and Heat Transfer
Calculations involving temperature change and phase transitions follow predictable patterns. Here’s a step-by-step guide to solving problems involving heat transfer:
- Determine if heat is being transferred. Ask: is there a temperature change, a phase change, or both?
- List all materials involved. Include their mass, initial and final temperatures, and phase.
- Identify what needs to be solved. Is it the amount of heat? Final temperature? Mass?
- List all known quantities. Use values given or ones that can be inferred.
- Select the correct equation:
- For temperature change:
[latex]Q = mc\Delta T[/latex]
- For phase change:
[latex]Q = mL[/latex]
Use [latex]L_{\text{f}}[/latex], [latex]L_{\text{v}}[/latex], or [latex]L_{\text{s}}[/latex] as appropriate.
- For temperature change:
- Substitute known values and calculate. If multiple steps are involved (e.g., heating, then melting), calculate each separately.
- Check your results. Do the numbers and units make sense? Ensure you’ve accounted for all relevant processes.
Check Your Understanding
Question: Why can snow remain on mountaintops even when daytime temperatures are above [latex]0^\circ\text{C}[/latex]?
Answer: Although the air may be warm enough, melting snow requires a significant amount of heat (latent heat of fusion). If that heat isn’t fully delivered—due to limited solar exposure, low air pressure, or reflective snow surface—the snow can persist despite above-freezing air temperatures.
Section Summary
- Substances exist in three phases: solid, liquid, and gas. Transitions between these phases are called phase changes.
- Heat transfer during a phase change does not alter temperature. Instead, it facilitates the breaking or forming of intermolecular bonds.
- The heat associated with a phase change is calculated as:
[latex]Q = mL[/latex]
where [latex]L[/latex] is the appropriate latent heat constant for fusion, vaporization, or sublimation.
Conceptual Questions
- Heat transfer can cause temperature and phase changes. What else can cause these changes?
- How does the latent heat of fusion of water help slow the decrease of air temperatures, perhaps preventing temperatures from falling significantly below [latex]\text{0ºC}[/latex], in the vicinity of large bodies of water?
- What is the temperature of ice right after it is formed by freezing water?
- If you place [latex]\text{0ºC}[/latex] ice into [latex]\text{0ºC}[/latex] water in an insulated container, what will happen? Will some ice melt, will more water freeze, or will neither take place?
- What effect does condensation on a glass of ice water have on the rate at which the ice melts? Will the condensation speed up the melting process or slow it down?
- In very humid climates where there are numerous bodies of water, such as in Florida, it is unusual for temperatures to rise above about [latex]\text{35º}\text{C}\left(\text{95º}\text{F}\right)[/latex]. In deserts, however, temperatures can rise far above this. Explain how the evaporation of water helps limit high temperatures in humid climates.
- In winters, it is often warmer in San Francisco than in nearby Sacramento, 150 km inland. In summers, it is nearly always hotter in Sacramento. Explain how the bodies of water surrounding San Francisco moderate its extreme temperatures.
- Putting a lid on a boiling pot greatly reduces the heat transfer necessary to keep it boiling. Explain why.
- Freeze-dried foods have been dehydrated in a vacuum. During the process, the food freezes and must be heated to facilitate dehydration. Explain both how the vacuum speeds up dehydration and why the food freezes as a result.
- When still air cools by radiating at night, it is unusual for temperatures to fall below the dew point. Explain why.
- In a physics classroom demonstration, an instructor inflates a balloon by mouth and then cools it in liquid nitrogen. When cold, the shrunken balloon has a small amount of light blue liquid in it, as well as some snow-like crystals. As it warms up, the liquid boils, and part of the crystals sublimate, with some crystals lingering for awhile and then producing a liquid. Identify the blue liquid and the two solids in the cold balloon. Justify your identifications using data from Table 102.1.
Problems & Exercises
- How much heat transfer (in kilocalories) is required to thaw a 0.450-kg package of frozen vegetables originally at [latex]0º\text{C}[/latex] if their heat of fusion is the same as that of water?
- A bag containing [latex]0º\text{C}[/latex] ice is much more effective in absorbing energy than one containing the same amount of [latex]0º\text{C}[/latex] water. a)How much heat transfer is necessary to raise the temperature of 0.800 kg of water from [latex]0º\text{C}[/latex] to [latex]\text{30}\text{.}0º\text{C?}[/latex] b)How much heat transfer is required to first melt 0.800 kg of [latex]0º\text{C}[/latex] ice and then raise its temperature? c) Explain how your answer supports the contention that the ice is more effective.
- (a) How much heat transfer is required to raise the temperature of a 0.750-kg aluminum pot containing 2.50 kg of water from [latex]\text{30}\text{.}0º\text{C}[/latex] to the boiling point and then boil away 0.750 kg of water? (b) How long does this take if the rate of heat transfer is 500 W [latex]1 \text{watt = 1 }\text{joule/second} \left(\text{1 W = 1 J/s}\right)[/latex]?
- The formation of condensation on a glass of ice water causes the ice to melt faster than it would otherwise. If 8.00 g of condensation forms on a glass containing both water and 200 g of ice, how many grams of the ice will melt as a result? Assume no other heat transfer occurs.
- On a trip, you notice that a 3.50-kg bag of ice lasts an average of one day in your cooler. What is the average power in watts entering the ice if it starts at [latex]0º\text{C}[/latex] and completely melts to [latex]0º\text{C}[/latex] water in exactly one day [latex]\text{1 watt = 1 joule/second}\phantom{\rule{0.25em}{0ex}} \left(\text{1 W = 1 J/s}\right)[/latex]?
- On a certain dry sunny day, a swimming pool’s temperature would rise by [latex]1\text{.}\text{50º}\text{C}[/latex] if not for evaporation. What fraction of the water must evaporate to carry away precisely enough energy to keep the temperature constant?
- (a) How much heat transfer is necessary to raise the temperature of a 0.200-kg piece of ice from [latex]-\text{20.}0º\text{C}[/latex] to [latex]\text{130º}\text{C}[/latex], including the energy needed for phase changes? (b) How much time is required for each stage, assuming a constant 20.0 kJ/s rate of heat transfer? (c) Make a graph of temperature versus time for this process.
- In 1986, a gargantuan iceberg broke away from the Ross Ice Shelf in Antarctica. It was approximately a rectangle 160 km long, 40.0 km wide, and 250 m thick. (a) What is the mass of this iceberg, given that the density of ice is [latex]\text{917}{\text{ kg/m}}^{3}[/latex]? (b) How much heat transfer (in joules) is needed to melt it? (c) How many years would it take sunlight alone to melt ice this thick, if the ice absorbs an average of [latex]\text{100 }{\text{W/m}}^{2}[/latex], 12.00 h per day?
- How many grams of coffee must evaporate from 350 g of coffee in a 100-g glass cup to cool the coffee from [latex]\text{95}\text{.}0º\text{C}[/latex] to [latex]\text{45}\text{.}0º\text{C}[/latex]? You may assume the coffee has the same thermal properties as water and that the average heat of vaporization is 2340 kJ/kg (560 cal/g). (You may neglect the change in mass of the coffee as it cools, which will give you an answer that is slightly larger than correct.)
- (a) It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases [latex]2\text{.}\text{80}×{\text{10}}^{7}\phantom{\rule{0.25em}{0ex}}\text{J}[/latex] of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released by burning 1.00 L of crude oil, if the water has its temperature raised from [latex]\text{20}\text{.}0º\text{C}[/latex] to [latex]\text{100º}\text{C}[/latex], it boils, and the resulting steam is raised to [latex]\text{300º}\text{C}[/latex]. (b) Discuss additional complications caused by the fact that crude oil has a smaller density than water.
- The energy released from condensation in thunderstorms can be very large. Calculate the energy released into the atmosphere for a small storm of radius 1 km, assuming that 1.0 cm of rain is precipitated uniformly over this area.
- To help prevent frost damage, 4.00 kg of [latex]0º\text{C}[/latex] water is sprayed onto a fruit tree. (a) How much heat transfer occurs as the water freezes? (b) How much would the temperature of the 200-kg tree decrease if this amount of heat transferred from the tree? Take the specific heat to be [latex]3\text{.35 kJ/kg}\cdot º\text{C}[/latex], and assume that no phase change occurs.
- A 0.250-kg aluminum bowl holding 0.800 kg of soup at [latex]\text{25}\text{.}0º\text{C}[/latex] is placed in a freezer. What is the final temperature if 377 kJ of energy is transferred from the bowl and soup, assuming the soup’s thermal properties are the same as that of water?
- A 0.0500-kg ice cube at [latex]-\text{30}\text{.}0º\text{C}[/latex] is placed in 0.400 kg of [latex]\text{35}\text{.}0º\text{C}[/latex] water in a very well-insulated container. What is the final temperature?
- If you pour 0.0100 kg of [latex]\text{20}\text{.}0º\text{C}[/latex] water onto a 1.20-kg block of ice (which is initially at [latex]-\text{15}\text{.}0º\text{C}[/latex]), what is the final temperature? You may assume that the water cools so rapidly that effects of the surroundings are negligible.
- Indigenous people sometimes cook in watertight baskets by placing hot rocks into water to bring it to a boil. What mass of [latex]\text{500º}\text{C}[/latex] rock must be placed in 4.00 kg of [latex]\text{15}\text{.}0º\text{C}[/latex] water to bring its temperature to [latex]\text{100º}\text{C}[/latex], if 0.0250 kg of water escapes as vapor from the initial sizzle? You may neglect the effects of the surroundings and take the average specific heat of the rocks to be that of granite.
- What would be the final temperature of the pan and water in Example 101.3 if 0.260 kg of water was placed in the pan and 0.0100 kg of the water evaporated immediately, leaving the remainder to come to a common temperature with the pan?
- In some countries, liquid nitrogen is used on dairy trucks instead of mechanical refrigerators. A 3.00-hour delivery trip requires 200 L of liquid nitrogen, which has a density of [latex]\text{808}{\text{ kg/m}}^{3}[/latex]. (a) Calculate the heat transfer necessary to evaporate this amount of liquid nitrogen and raise its temperature to [latex]3\text{.}\text{00º}\text{C}[/latex]. (Use [latex]{c}_{p}[/latex] and assume it is constant over the temperature range.) This value is the amount of cooling the liquid nitrogen supplies. (b) What is this heat transfer rate in kilowatt-hours? (c) Compare the amount of cooling obtained from melting an identical mass of [latex]0º\text{C}[/latex] ice with that from evaporating the liquid nitrogen.
- Some gun fanciers make their own bullets, which involves melting and casting the lead slugs. How much heat transfer is needed to raise the temperature and melt 0.500 kg of lead, starting from [latex]\text{25}\text{.}0º\text{C}[/latex]?
Footnotes
- 1 Values quoted at the normal melting and boiling temperatures at standard atmospheric pressure (1 atm).
- 2 At [latex]\text{37}\text{.}0º\text{C}[/latex] (body temperature), the heat of vaporization [latex]{L}_{v}[/latex] for water is 2430 kJ/kg or 580 kcal/kg
- 3 At [latex]\text{37.}0º\text{C}[/latex] (body temperature), the heat of vaporization [latex]{L}_{v}[/latex] for water is 2430 kJ/kg or 580 kcal/kg
Glossary
- heat of sublimation
- the energy required to change a substance from the solid phase to the vapor phase
- latent heat coefficient
- a physical constant equal to the amount of heat transferred for every 1 kg of a substance during the change in phase of the substance
- sublimation
- the transition from the solid phase to the vapor phase
a physical constant equal to the amount of heat transferred for every 1 kg of a substance during the change in phase of the substance
the temperature at which relative humidity is 100%; the temperature at which water starts to condense out of the air
the phase change from solid to gas
the energy required to change a substance from the solid phase to the vapor phase


