Temperature, Kinetic Theory, and the Gas Laws
98 Humidity, Evaporation, and Boiling
Learning Objectives
- Explain the relationship between vapor pressure of water and the capacity of air to contain water vapor.
- Describe the connection between relative humidity and the partial pressure of water vapor in the air.
- Calculate vapor density using known vapor pressure values.
- Calculate humidity and dew point.

The common phrase “It’s not the heat, it’s the humidity” highlights a key physiological principle. Our bodies cool themselves by evaporating sweat from the skin and releasing moisture through respiration. In humid conditions, evaporation is suppressed, making it harder for the body to regulate internal temperature, leading to heat stress. Conversely, very dry conditions can dry out mucous membranes, increasing susceptibility to respiratory infections.
When we refer to “humidity,” we are usually referring to relative humidity, which compares the current amount of water vapor in the air to the maximum possible amount at a given temperature. At 100% relative humidity—also called saturation—evaporation stops, and any cooling will result in condensation. This is why fog or dew can form when temperatures fall to the dew point.
The dew point is the temperature at which air becomes saturated and water vapor condenses. If air cools to this point overnight, dew forms on surfaces such as grass or leaves. Hair dryers and other heated airflow devices dry surfaces more effectively because the higher temperature increases molecular kinetic energy and, in turn, the evaporation rate.
Evaporation and condensation are governed by the vapor pressure of water. At any given temperature, water molecules with enough kinetic energy escape into the gas phase. As shown in Figure 98.2, even below boiling, water molecules can evaporate. In a sealed container, evaporation continues until the gas phase contains enough molecules to reach equilibrium—when the rate of condensation equals the rate of evaporation. The resulting pressure exerted by water vapor is the vapor pressure, and it increases with temperature because molecular motion is more vigorous.
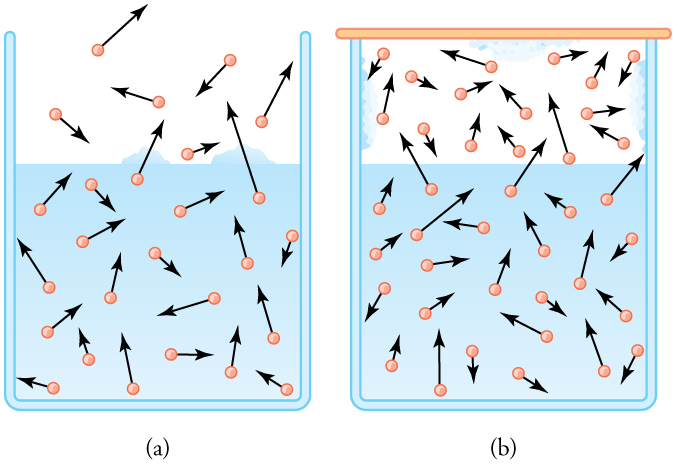
Relative humidity can be understood in terms of partial pressure. At 100% relative humidity, the partial pressure of water vapor equals the vapor pressure of water at that temperature. If the partial pressure is less than the vapor pressure, more evaporation can occur. If the partial pressure is greater than the vapor pressure, condensation happens. Importantly, air doesn’t “hold” water vapor—this is a misconception. The capacity for water vapor is dictated by the vapor pressure of water and temperature, not the chemical properties of air. Table 98.1 gives representative values of water vapor pressure over a range of temperatures.
Example 98.1: Calculating Density Using Vapor Pressure
Table 98.1 gives the vapor pressure of water at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex] as [latex]2\text{.}\text{33}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}\text{Pa}\text{.}[/latex] Use the ideal gas law to calculate the density of water vapor in [latex]\text{g}/{\text{m}}^{3}[/latex] that would create a partial pressure equal to this vapor pressure. Compare the result with the saturation vapor density given in the table.
Strategy
To solve this problem, we need to break it down into a two steps. The partial pressure follows the ideal gas law,
where [latex]n[/latex] is the number of moles. If we solve this equation for [latex]n/V[/latex] to calculate the number of moles per cubic meter, we can then convert this quantity to grams per cubic meter as requested. To do this, we need to use the molecular mass of water, which is given in the periodic table.
Solution
- Identify the knowns and convert them to the proper units:
- temperature [latex]T=\text{20}\text{º}\text{C=293 K}[/latex]
- vapor pressure [latex]P[/latex] of water at [latex]\text{20}\text{º}\text{C}[/latex] is [latex]2\text{.}\text{33}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}\text{Pa}[/latex]
- molecular mass of water is [latex]\text{18}\text{.}0\phantom{\rule{0.25em}{0ex}}\text{g/mol}[/latex]
- Solve the ideal gas law for [latex]n/V[/latex].
[latex]\frac{n}{V}=\frac{P}{\text{RT}}[/latex] - Substitute known values into the equation and solve for [latex]n/V[/latex].
[latex]\frac{n}{V}=\frac{P}{\text{RT}}=\frac{2\text{.}\text{33}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}\text{Pa}}{\left(8\text{.}\text{31}\phantom{\rule{0.25em}{0ex}}\text{J/mol}\cdot \text{K}\right)\left(\text{293}\phantom{\rule{0.25em}{0ex}}\text{K}\right)}=0\text{.}\text{957}\phantom{\rule{0.25em}{0ex}}{\text{mol/m}}^{3}[/latex] - Convert the density in moles per cubic meter to grams per cubic meter.
[latex]\rho =\left(0\text{.}\text{957}\frac{\text{mol}}{{\text{m}}^{3}}\right)\left(\frac{\text{18}\text{.}\text{0 g}}{\text{mol}}\right)=\text{17}\text{.}2\phantom{\rule{0.25em}{0ex}}{\text{g/m}}^{3}[/latex]
Discussion
The density is obtained by assuming a pressure equal to the vapor pressure of water at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex]. The density found is identical to the value in Table 98.1, which means that a vapor density of [latex]\text{17}\text{.}2\phantom{\rule{0.25em}{0ex}}{\text{g/m}}^{3}[/latex] at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex] creates a partial pressure of [latex]2\text{.}\text{33}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}\text{Pa,}[/latex] equal to the vapor pressure of water at that temperature. If the partial pressure is equal to the vapor pressure, then the liquid and vapor phases are in equilibrium, and the relative humidity is 100%. Thus, there can be no more than 17.2 g of water vapor per [latex]{\text{m}}^{3}[/latex] at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex], so that this value is the saturation vapor density at that temperature. This example illustrates how water vapor behaves like an ideal gas: the pressure and density are consistent with the ideal gas law (assuming the density in the table is correct). The saturation vapor densities listed in Table 98.1 are the maximum amounts of water vapor that air can hold at various temperatures.
Percent Relative Humidity
Relative humidity is a measure of how much water vapor is in the air compared to the maximum amount the air could contain at a specific temperature. One common way to express this is through percent relative humidity, which is the ratio of the actual water vapor density in the air to the maximum (or saturation) vapor density possible at that temperature.
The formula for percent relative humidity is:
This ratio tells us how close the air is to being fully saturated. At 100% relative humidity, the vapor density is equal to the saturation vapor density, and no more water can evaporate into the air. At lower values, more evaporation can still occur.
We can use this relationship along with data from Table 98.1 to calculate relative humidity or vapor density under various environmental conditions. This has important implications for human health, comfort, and physiological regulation of water loss through respiration and skin.
Example 98.2: Calculating Humidity and Dew Point
(a) Calculate the percent relative humidity on a day when the temperature is [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] and the air contains 9.40 g of water vapor per [latex]{\text{m}}^{3}[/latex]. (b) At what temperature will this air reach 100% relative humidity (the saturation density)? This temperature is the dew point. (c) What is the humidity when the air temperature is [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] and the dew point is [latex]–\text{10}\text{.}0\text{º}\text{C}[/latex]?
Strategy and Solution
- Percent relative humidity is defined as the ratio of vapor density to saturation vapor density.
[latex]\text{percent relative humidity}=\frac{\text{vapor density}}{\text{saturation vapor density}}×\text{100}[/latex]
The first is given to be [latex]9\text{.}{\text{40 g/m}}^{3}[/latex], and the second is found in Table 98.1 to be [latex]\text{23}\text{.}{\text{0 g/m}}^{3}[/latex]. Thus,
[latex]\text{percent relative humidity}=\frac{9\text{.}{\text{40 g/m}}^{3}}{\text{23}\text{.}{\text{0 g/m}}^{3}}×\text{100}=\text{40}\text{.}9\text{}\text{.%}[/latex] - The air contains [latex]9\text{.}{\text{40 g/m}}^{3}[/latex] of water vapor. The relative humidity will be 100% at a temperature where [latex]9\text{.}{\text{40 g/m}}^{3}[/latex] is the saturation density. Inspection of Table 98.1 reveals this to be the case at [latex]\text{10}\text{.}0\text{º}\text{C}[/latex], where the relative humidity will be 100%. That temperature is called the dew point for air with this concentration of water vapor.
- Here, the dew point temperature is given to be [latex]–\text{10}\text{.}0\text{º}\text{C}[/latex]. Using Table 98.1, we see that the vapor density is [latex]2\text{.}{\text{36 g/m}}^{3}[/latex], because this value is the saturation vapor density at [latex]–\text{10}\text{.}0\text{º}\text{C}[/latex]. The saturation vapor density at [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] is seen to be [latex]\text{23}\text{.}{\text{0 g/m}}^{3}[/latex]. Thus, the relative humidity at [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] is
[latex]\text{percent relative humidity}=\frac{2\text{.}{\text{36 g/m}}^{3}}{\text{23}\text{.}{\text{0 g/m}}^{3}}×\text{100}=\text{10}\text{.}3\text{%}\text{}\text{.}[/latex]
Discussion
The importance of dew point is that air temperature cannot drop below [latex]\text{10}\text{.}0\text{º}\text{C}[/latex] in part (b), or [latex]–\text{10}\text{.}0\text{º}\text{C}[/latex] in part (c), without water vapor condensing out of the air. If condensation occurs, considerable transfer of heat occurs (discussed in Heat and Heat Transfer Methods), which prevents the temperature from further dropping. When dew points are below [latex]0\text{ºC}[/latex], freezing temperatures are a greater possibility, which explains why farmers keep track of the dew point. Low humidity in deserts means low dew-point temperatures. Thus condensation is unlikely. If the temperature drops, vapor does not condense in liquid drops. Because no heat is released into the air, the air temperature drops more rapidly compared to air with higher humidity. Likewise, at high temperatures, liquid droplets do not evaporate, so that no heat is removed from the gas to the liquid phase. This explains the large range of temperature in arid regions.
Boiling and Vapor Pressure
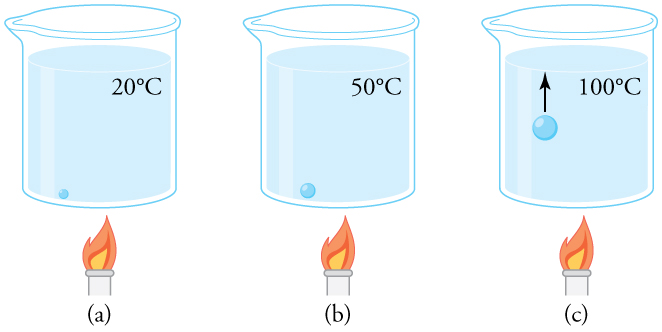
Why does water boil at [latex]\text{100ºC}[/latex] under standard atmospheric pressure? If we refer to Table 98.1, we see that at [latex]\text{100ºC}[/latex], the vapor pressure of water is [latex]1.01 \times 10^5 \ \text{Pa}[/latex], or 1.00 atm. This means that at this temperature, the water’s vapor pressure equals the surrounding atmospheric pressure, allowing it to evaporate freely and form bubbles inside the liquid. But what exactly causes those bubbles?
Water contains dissolved gases such as oxygen and nitrogen, which form small air bubbles, especially at the bottom of a heated container. At a starting temperature of [latex]\text{20ºC}[/latex], a bubble near the bottom of the container contains some water vapor (about 2.30%) and mostly air. The total pressure inside the bubble is approximately 1.00 atm, the same as the surrounding water (ignoring small pressure variations due to depth).
As the temperature increases, more water vapor enters the bubble, raising the partial pressure of the vapor. To maintain the total pressure at 1.00 atm, the bubble expands. At [latex]\text{100ºC}[/latex], the water’s vapor pressure matches the atmospheric pressure, allowing vapor to enter the bubble continuously. As a result, the bubble grows large enough to rise through the liquid due to increased buoyant force. When it reaches the surface and escapes, we observe this as boiling. (See Figure 98.3.)
Freeze drying is a method of preserving food and other biological samples. In this process, the material is placed in a vacuum chamber and both the pressure and temperature are lowered. How does reducing the pressure speed up drying, and why does the food freeze?
When atmospheric pressure is reduced, the partial pressure of water vapor also decreases. This lowers the relative humidity, allowing water to evaporate more readily from the sample. The molecules that evaporate are the ones with the highest kinetic energy. As these escape, the average kinetic energy (and thus the temperature) of the remaining molecules drops, leading to cooling. Eventually, the sample freezes while continuing to lose water—a phenomenon known as freeze drying.
PhET Explorations: States of Matter
Use this interactive simulation to observe how solids, liquids, and gases behave at the molecular level under different conditions. By adding or removing thermal energy, you can explore how particles gain or lose kinetic energy and how this affects phase transitions.
Change the temperature or volume of the system and observe corresponding changes in pressure and particle motion. Watch a pressure-temperature diagram update in real time to reinforce the relationships among thermodynamic variables. This tool is especially useful for visualizing how molecular interactions lead to macroscopic properties like pressure and phase state.
Section Summary
- Relative humidity is the fraction of water vapor in the air compared to the saturation value for that temperature.
- The saturation vapor density is determined from water’s vapor pressure at a given temperature.
- Percent relative humidity is calculated as:
[latex]\text{Percent Relative Humidity} = \frac{\text{Vapor Density}}{\text{Saturation Vapor Density}} \times 100[/latex]
- The dew point is the temperature at which the air becomes saturated (100% relative humidity), causing condensation.
Conceptual Questions
- Because humidity depends only on water’s vapor pressure and temperature, are the saturation vapor densities listed in Table 98.1 valid in an atmosphere of helium at a pressure of [latex]1\text{.}\text{01}×{\text{10}}^{5}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex], rather than air? Are those values affected by altitude on Earth?
- Why does a beaker of [latex]\text{40}\text{.}0\text{º}\text{C}[/latex] water placed in a vacuum chamber start to boil as the chamber is evacuated (air is pumped out of the chamber)? At what pressure does the boiling begin? Would food cook any faster in such a beaker?
- Why does rubbing alcohol evaporate much more rapidly than water at STP (standard temperature and pressure)?
Problems & Exercises
- Dry air is 78.1% nitrogen. What is the partial pressure of nitrogen when the atmospheric pressure is [latex]1\text{.}\text{01}×{\text{10}}^{5}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex]?
- (a) What is the vapor pressure of water at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex]? (b) What percentage of atmospheric pressure does this correspond to? (c) What percent of [latex]\text{20}\text{.}0\text{º}\text{C}[/latex] air is water vapor if it has 100% relative humidity? (The density of dry air at [latex]\text{20}\text{.}0\text{º}\text{C}[/latex] is [latex]1\text{.}\text{20}\phantom{\rule{0.25em}{0ex}}{\text{kg/m}}^{3}[/latex].)
- Pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. (a) What pressure is necessary to raise the boiling point to [latex]\text{120}\text{.}0\text{º}\text{C}[/latex]? (b) What gauge pressure does this correspond to?
- (a) At what temperature does water boil at an altitude of 1500 m (about 5000 ft) on a day when atmospheric pressure is [latex]8\text{.}\text{59}×{\text{10}}^{4}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}\text{?}[/latex] (b) What about at an altitude of 3000 m (about 10,000 ft) when atmospheric pressure is [latex]7\text{.}\text{00}×{\text{10}}^{4}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}\text{?}[/latex]
- What is the atmospheric pressure on top of Mt. Everest on a day when water boils there at a temperature of [latex]\text{70}\text{.}0\text{º}\text{C?}[/latex]
- At a spot in the high Andes, water boils at [latex]\text{80}\text{.}0\text{º}\text{C}[/latex], greatly reducing the cooking speed of potatoes, for example. What is atmospheric pressure at this location?
- What is the relative humidity on a [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] day when the air contains [latex]\text{18}\text{.}0\phantom{\rule{0.25em}{0ex}}{\text{g/m}}^{3}[/latex] of water vapor?
- What is the density of water vapor in [latex]{\text{g/m}}^{3}[/latex] on a hot dry day in the desert when the temperature is [latex]\text{40}\text{.}0\text{º}\text{C}[/latex] and the relative humidity is 6.00%
- A deep-sea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of [latex]1\text{.}\text{01}×{\text{10}}^{5}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex]. (a) What is the partial pressure of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of [latex]2\text{.}\text{00}×{\text{10}}^{6}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex], what percent oxygen should it be to have the same oxygen partial pressure as at sea level?
- The vapor pressure of water at [latex]\text{40}\text{.}0\text{º}\text{C}[/latex] is [latex]7\text{.}\text{34}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex]. Using the ideal gas law, calculate the density of water vapor in [latex]{\text{g/m}}^{3}[/latex] that creates a partial pressure equal to this vapor pressure. The result should be the same as the saturation vapor density at that temperature [latex]\left(\text{51}\text{.}{\text{1 g/m}}^{3}\right)\text{.}[/latex
- Air in human lungs has a temperature of [latex]\text{37}\text{.}0\text{º}\text{C}[/latex] and a saturation vapor density of [latex]\text{44}\text{.}{\text{0 g/m}}^{3}[/latex]. (a) If 2.00 L of air is exhaled and very dry air inhaled, what is the maximum loss of water vapor by the person? (b) Calculate the partial pressure of water vapor having this density, and compare it with the vapor pressure of [latex]6\text{.}\text{31}×{\text{10}}^{3}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex].
- If the relative humidity is 90.0% on a muggy summer morning when the temperature is [latex]\text{20}\text{.}0\text{º}\text{C}[/latex], what will it be later in the day when the temperature is [latex]\text{30}\text{.}0\text{º}\text{C}[/latex], assuming the water vapor density remains constant?
- Late on an autumn day, the relative humidity is 45.0% and the temperature is [latex]\text{20}\text{.}0\text{º}\text{C}[/latex]. What will the relative humidity be that evening when the temperature has dropped to [latex]\text{10}\text{.}0\text{º}\text{C}[/latex], assuming constant water vapor density?
- Atmospheric pressure atop Mt. Everest is [latex]3\text{.}\text{30}×{\text{10}}^{4}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex]. (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percent oxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is [latex]1\text{.}\text{01}×{\text{10}}^{5}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}\text{?}[/latex] (c) One of the most severe problems for those climbing very high mountains is the extreme drying of breathing passages. Why does this drying occur?
- What is the dew point (the temperature at which 100% relative humidity would occur) on a day when relative humidity is 39.0% at a temperature of [latex]\text{20}\text{.}0\text{º}\text{C}[/latex]?
- On a certain day, the temperature is [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] and the relative humidity is 90.0%. How many grams of water must condense out of each cubic meter of air if the temperature falls to [latex]\text{15}\text{.}0\text{º}\text{C}[/latex]? Such a drop in temperature can, thus, produce heavy dew or fog.
- Integrated Concepts The boiling point of water increases with depth because pressure increases with depth. At what depth will fresh water have a boiling point of [latex]\text{150}\text{º}\text{C}[/latex], if the surface of the water is at sea level?
- Integrated Concepts (a) At what depth in fresh water is the critical pressure of water reached, given that the surface is at sea level? (b) At what temperature will this water boil? (c) Is a significantly higher temperature needed to boil water at a greater depth
- Integrated Concepts To get an idea of the small effect that temperature has on Archimedes’ principle, calculate the fraction of a copper block’s weight that is supported by the buoyant force in [latex]0\text{º}\text{C}[/latex] water and compare this fraction with the fraction supported in [latex]\text{95}\text{.}0\text{º}\text{C}[/latex] water.
- Integrated Concepts If you want to cook in water at [latex]\text{150}\text{º}\text{C}[/latex], you need a pressure cooker that can withstand the necessary pressure. (a) What pressure is required for the boiling point of water to be this high? (b) If the lid of the pressure cooker is a disk 25.0 cm in diameter, what force must it be able to withstand at this pressure.
- Unreasonable Results (a) How many moles per cubic meter of an ideal gas are there at a pressure of [latex]1\text{.}\text{00}×{\text{10}}^{\text{14}}\phantom{\rule{0.25em}{0ex}}{\text{N/m}}^{2}[/latex] and at [latex]0\text{º}\text{C}[/latex]? (b) What is unreasonable about this result? (c) Which premise or assumption is responsible?
- Unreasonable Results (a) An automobile mechanic claims that an aluminum rod fits loosely into its hole on an aluminum engine block because the engine is hot and the rod is cold. If the hole is 10.0% bigger in diameter than the [latex]\text{22}\text{.}0\text{º}\text{C}[/latex] rod, at what temperature will the rod be the same size as the hole? (b) What is unreasonable about this temperature? (c) Which premise is responsible?
- Unreasonable Results The temperature inside a supernova explosion is said to be [latex]2\text{.}\text{00}×{\text{10}}^{\text{13}}\phantom{\rule{0.25em}{0ex}}\text{K}[/latex]. (a) What would the average velocity [latex]{v}_{\text{rms}}[/latex] of hydrogen atoms be? (b) What is unreasonable about this velocity? (c) Which premise or assumption is responsible?
- Unreasonable Results Suppose the relative humidity is 80% on a day when the temperature is [latex]\text{30}\text{.}0\text{º}\text{C}[/latex]. (a) What will the relative humidity be if the air cools to [latex]\text{25}\text{.}0\text{º}\text{C}[/latex] and the vapor density remains constant? (b) What is unreasonable about this result? (c) Which premise is responsible?
Glossary
- dew point
- the temperature at which relative humidity is 100%; the temperature at which water starts to condense out of the air
- saturation
- the condition of 100% relative humidity
- percent relative humidity
- the ratio of vapor density to saturation vapor density
- relative humidity
- the amount of water in the air relative to the maximum amount the air can hold
the amount of water in the air relative to the maximum amount the air can hold
the temperature at which relative humidity is 100%; the temperature at which water starts to condense out of the air
the condition of 100% relative humidity
the ratio of vapor density to saturation vapor density

