Heat and Heat Transfer Methods
100 Heat
Learning Objectives
- Define heat as a mode of energy transfer due to temperature difference.
In the chapter on Work, Energy, and Energy Resources, we learned that doing work on an object—applying force through a distance—transfers energy, often in the form of mechanical energy. In Temperature, Kinetic Theory, and the Gas Laws, we saw that temperature is proportional to the average kinetic energy of atoms or molecules. A system at higher temperature has greater internal energy due to this microscopic motion.
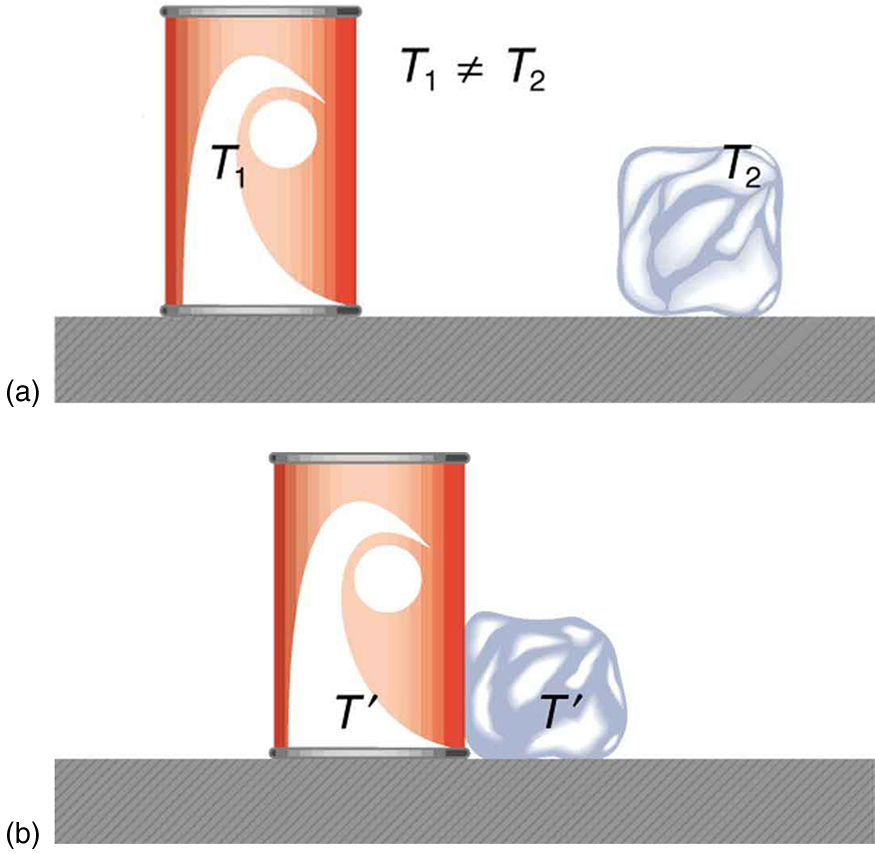
When two objects at different temperatures are brought into contact, energy spontaneously flows from the hotter object to the cooler one. No mechanical work is done—no force acts through a distance—but energy is transferred simply because of the temperature difference. This transfer of energy is defined as heat.
Heat is therefore the energy transferred due solely to a temperature difference between systems. Once the two objects reach the same temperature—known as thermal equilibrium—heat transfer ceases.
This leads to a common misconception: heat is not the same as temperature. People often say things like “the heat was unbearable” when they really mean the temperature was high. Heat refers to the transfer of energy, not the thermal state of an object.
Units of Heat
Since heat is energy, it is measured in joules (J) in the SI system. Another commonly used unit is the calorie (cal), defined as the energy required to raise the temperature of 1 gram of water by 1ºC, typically from 14.5ºC to 15.5ºC. A more practical unit in biology and nutrition is the kilocalorie (kcal), which is the energy required to raise the temperature of 1 kilogram of water by 1ºC. This is the same unit used in food labels, where 1 Calorie (with a capital C) = 1 kilocalorie = 1000 calories.
Mechanical Equivalent of Heat
In the 1800s, James Prescott Joule demonstrated that work—like turning a paddle in water—can also increase temperature. He showed that work and heat are both forms of energy transfer and can produce identical effects. This led to the concept of the mechanical equivalent of heat, the amount of mechanical work needed to produce the same energy transfer as heat.
This equivalence tells us that energy is conserved regardless of the transfer method—work or heat—and both can increase a system’s internal energy.
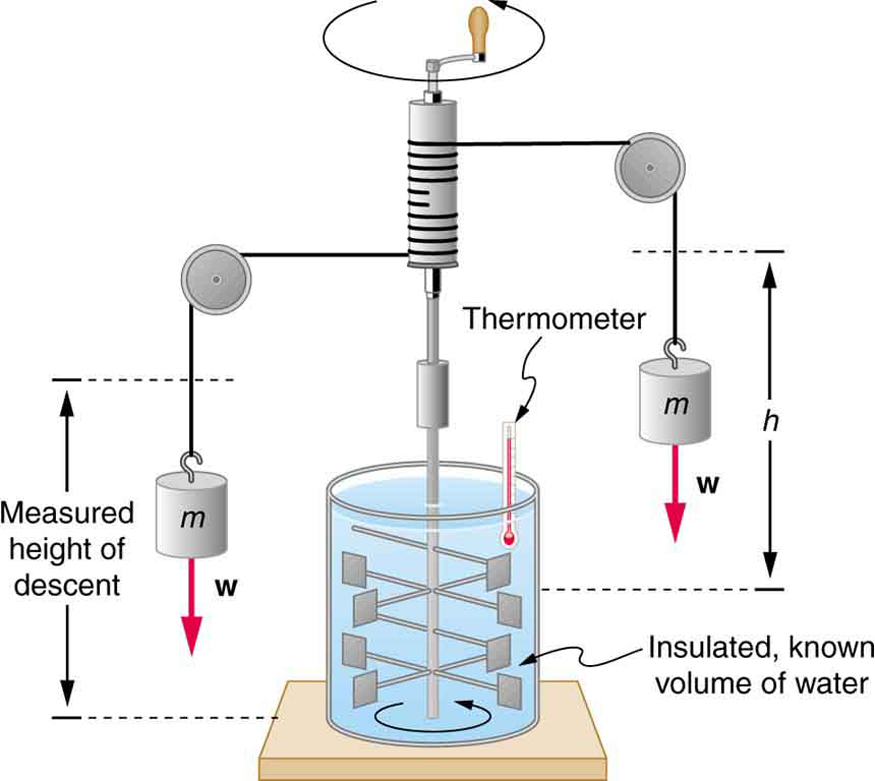
Joule’s insight helped establish the principle of conservation of energy, a foundational concept in all physical sciences. Importantly, it showed that internal energy can be increased not only by adding heat but also by performing work. However, while a system has a well-defined internal energy, it does not have a specific “heat content.” Heat only exists during transfer.
Check Your Understanding
Question: Two samples (A and B) of the same substance are placed in a lab. One receives 10 kJ of heat; the other has 10 kJ of work done on it. Can you tell which one received heat?
Answer: No. Both processes transfer energy into the system, increasing internal energy. Since only internal energy matters for the final state, there is no physical way to distinguish between heat or work as the source.
Summary
- Heat and work are the two primary methods of energy transfer.
- Heat is the energy transferred due to a temperature difference between objects.
- Units of heat include the joule (J) and kilocalorie (kcal).
- 1 kcal is defined as the energy required to raise 1.00 kg of water by 1.00ºC.
- The mechanical equivalent of heat is given by [latex]1.00 \ \text{kcal} = 4186 \ \text{J}[/latex].
Conceptual Questions
- How is heat transfer related to temperature?
- Describe a situation in which heat transfer occurs. What are the resulting forms of energy?
- When heat transfers into a system, is the energy stored as heat? Explain briefly.
Glossary
- heat
- the spontaneous transfer of energy due to a temperature difference
- kilocalorie
- [latex]\phantom{\rule{0.25em}{0ex}}1\phantom{\rule{0.25em}{0ex}}\text{kilocalorie}\phantom{\rule{0.25em}{0ex}}\text{=}\phantom{\rule{0.25em}{0ex}}\text{1000}\phantom{\rule{0.25em}{0ex}}\text{calories}[/latex]
- mechanical equivalent of heat
- the work needed to produce the same effects as heat transfer
the spontaneous transfer of energy due to a temperature difference
the energy an object has by reason of its motion, equal to [latex]\frac{1}{2}{\text{mv}}^{2}[/latex] for the translational (i.e., non-rotational) motion of an object of mass [latex]m[/latex] moving at speed [latex]v[/latex]
the condition in which heat no longer flows between two objects that are in contact; the two objects have the same temperature
the quantity measured by a thermometer
SI unit of work and energy, equal to one newton-meter
the transfer of energy by a force that causes an object to be displaced; the product of the component of the force in the direction of the displacement and the magnitude of the displacement
the general law that total energy is constant in any process; energy may change in form or be transferred from one system to another, but the total remains the same
[latex]\phantom{\rule{0.25em}{0ex}}1\phantom{\rule{0.25em}{0ex}}\text{kilocalorie}\phantom{\rule{0.25em}{0ex}}\text{=}\phantom{\rule{0.25em}{0ex}}\text{1000}\phantom{\rule{0.25em}{0ex}}\text{calories}[/latex]
the work needed to produce the same effects as heat transfer

