Heat and Heat Transfer Methods
105 Convection
Learning Objectives
- Describe the process of heat transfer by convection.
- Explain biological and environmental examples of convection.
Convection is a method of heat transfer that involves the macroscopic movement of fluids—liquids or gases. It occurs when regions of different temperatures create density differences that cause fluid to move. This process transports thermal energy from one region to another. Convection plays a central role in both natural environments and biological systems.
For example, in the human body, the circulatory system regulates heat by varying blood flow to the skin. When the body overheats, surface blood vessels dilate (widen), increasing blood flow to the skin where excess heat is lost through sweat evaporation. When it’s cold, these vessels constrict, preserving internal heat. The body also loses heat through the air we exhale—a continuous example of convective heat exchange.
On a larger scale, convection drives global weather and ocean patterns. Heated air from equatorial regions rises and flows toward the poles, while cooler polar air sinks and flows toward the equator. These convective loops regulate Earth’s climate. A similar process occurs in car engines: water circulates through the engine to remove heat, helping maintain optimal temperatures for performance.
Natural convection arises from buoyancy. As a fluid heats up, its molecules move faster and spread apart, lowering its density. The warmer, less dense fluid rises, while the cooler, denser fluid sinks, creating a circulation pattern. This is how hot air rises in a room or how water circulates in a boiling pot.
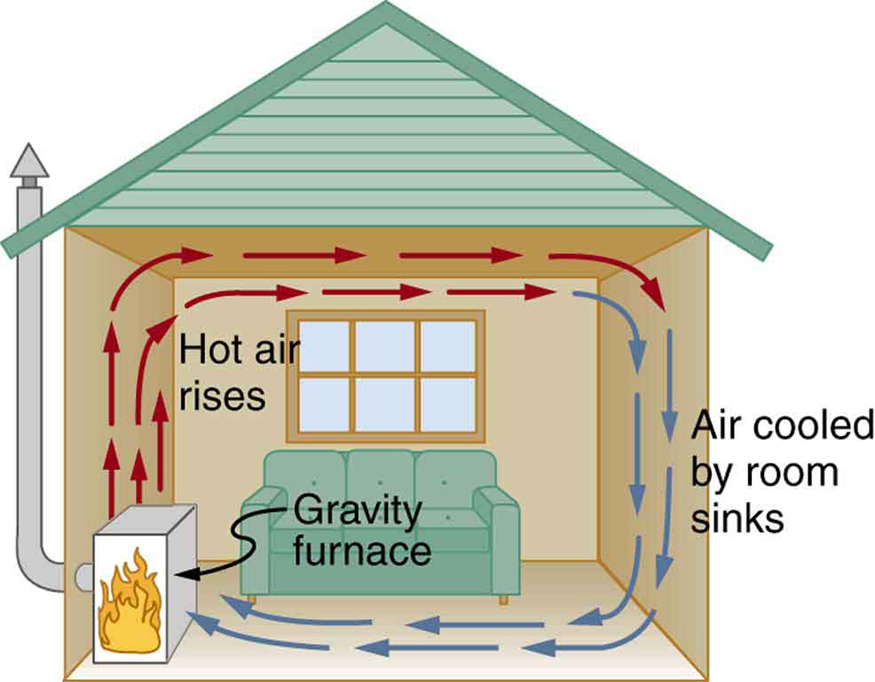
Consider the home heating system shown in Figure 105.1. Air warmed by a furnace becomes less dense and rises. As it cools near the ceiling and walls, it becomes denser and sinks back toward the furnace. This continuous cycle of rising and sinking air forms a convective loop that efficiently distributes heat throughout the space.
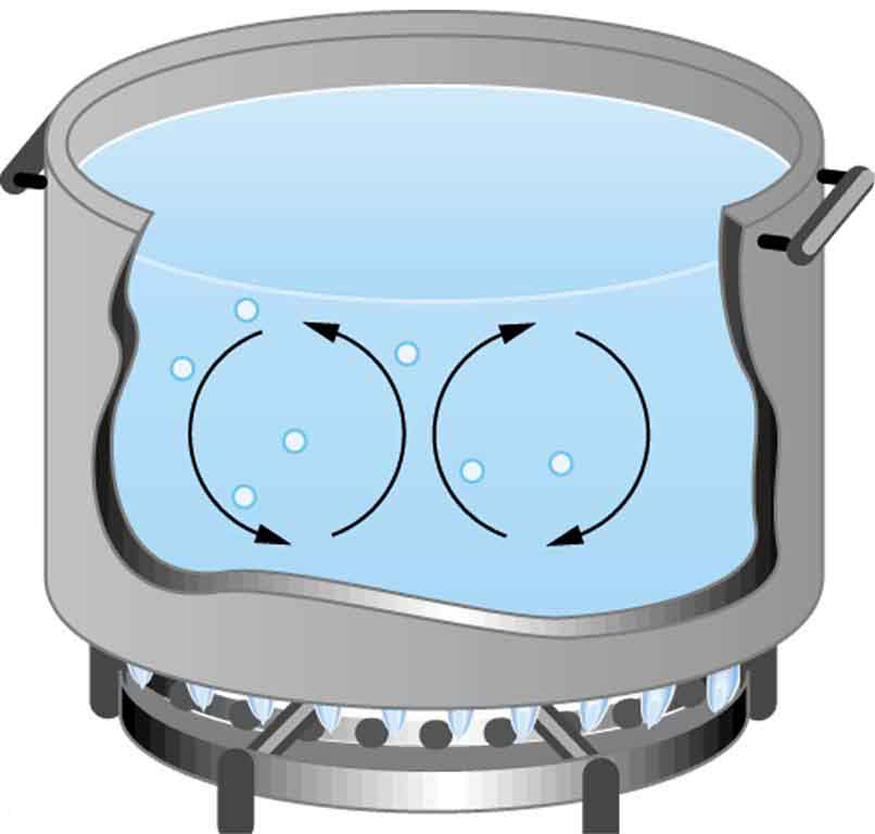
Convection also occurs in water. In Figure 105.2, water heated at the bottom of a pot becomes less dense and rises, while cooler water sinks. These movements form convective currents that distribute heat throughout the pot. This same principle applies to body fluids such as cerebrospinal fluid or blood plasma when heated locally—for example, by inflammation or therapeutic heat packs.
Take-Home Experiment: Convection Rolls in a Heated Pan
Try this experiment to visualize convection in action:
- Fill two small pots with water.
- Add a drop of food coloring near the bottom of each using an eyedropper.
- Place one pot on a counter (no heat) and the other on a stovetop over low heat.
- Observe how the food coloring spreads over time in each pot.
- Note how convective loops carry the color to the top in the heated pot, while little to no movement occurs in the unheated one.
Example 105.1 Calculating Heat Transfer by Convection: Convection of Air Through the Walls of a House
Most houses are not airtight: air goes in and out around doors and windows, through cracks and crevices, following wiring to switches and outlets, and so on. The air in a typical house is completely replaced in less than an hour. Suppose that a moderately-sized house has inside dimensions [latex]12.0\text{m}×18.0\text{m}×3.00\text{m}[/latex]
high, and that all air is replaced in 30.0 min. Calculate the heat transfer per unit time in watts needed to warm the incoming cold air by [latex]\text{10}\text{.}0º\text{C}[/latex], thus replacing the heat transferred by convection alone.
Strategy
Heat is used to raise the temperature of air so that [latex]Q=\text{mc}\text{Δ}T[/latex]. The rate of heat transfer is then [latex]Q/t[/latex], where [latex]t[/latex] is the time for air turnover. We are given that [latex]\text{Δ}T[/latex]
is [latex]\text{10}\text{.}0º\text{C}[/latex], but we must still find values for the mass of air and its specific heat before we can calculate [latex]Q[/latex]. The specific heat of air is a weighted average of the specific heats of nitrogen and oxygen, which gives [latex]c={c}_{\text{p}}\cong \text{1000}\text{ J/kg}\cdot º\text{C}[/latex] from Table 105.1 (note that the specific heat at constant pressure must be used for this process).
Solution
- Determine the mass of air from its density and the given volume of the house. The density is given from the density [latex]\rho[/latex] and the volume
[latex]m=\text{ρV}=\left(1\text{.}\text{29}\phantom{\rule{0.25em}{0ex}}{\text{kg/m}}^{3}\right)\left(\text{12}\text{.}0\phantom{\rule{0.25em}{0ex}}\text{m}×\text{18}\text{.}0\phantom{\rule{0.25em}{0ex}}\text{m}×3.00\phantom{\rule{0.25em}{0ex}}\text{m}\right)=\text{836}\phantom{\rule{0.25em}{0ex}}\text{kg.}[/latex]
- Calculate the heat transferred from the change in air temperature: [latex]Q=\text{mc}\text{Δ}T[/latex] so that
[latex]Q=\left(\text{836}\phantom{\rule{0.25em}{0ex}}\text{kg}\right)\left(\text{1000 J/kg}\cdot º\text{C}\right)\left(\text{10.0º}\text{C}\right)\text{= 8}\text{.}\text{36}×{\text{10}}^{6}\phantom{\rule{0.25em}{0ex}}\text{J.}[/latex]
- Calculate the heat transfer from the heat [latex]Q[/latex] and the turnover time [latex]t[/latex]. Since air is turned over in [latex]t=0\text{.}500\phantom{\rule{0.25em}{0ex}}\text{h}=\text{1800}\phantom{\rule{0.25em}{0ex}}\text{s}[/latex], the heat transferred per unit time is
[latex]\frac{Q}{t}=\frac{8\text{.}\text{36}×{\text{10}}^{6 }\text{J}}{\text{1800 }\text{s}}=4\text{.}64\phantom{\rule{0.25em}{0ex}}\text{kW.}[/latex]
Discussion
This rate of heat transfer is equal to the power consumed by about forty-six 100-W light bulbs. Newly constructed homes are designed for a turnover time of 2 hours or more, rather than 30 minutes for the house of this example. Weather stripping, caulking, and improved window seals are commonly employed. More extreme measures are sometimes taken in very cold (or hot) climates to achieve a tight standard of more than 6 hours for one air turnover. Still longer turnover times are unhealthy, because a minimum amount of fresh air is necessary to supply oxygen for breathing and to dilute household pollutants. The term used for the process by which outside air leaks into the house from cracks around windows, doors, and the foundation is called “air infiltration.”
Convection becomes especially important when considering how heat is transferred in the environment and in biological systems. For example, on a cold, windy day, we often feel much colder than the actual air temperature suggests. This is due to wind chill—a result of convective heat transfer that enhances the rate at which heat leaves the body. When moving air comes into contact with our skin, it replaces the warm air layer that normally insulates us, increasing the heat loss through both convection and conduction.
The table below shows approximate wind-chill factors. These values represent the temperature of still air that would result in the same rate of heat loss as the combination of moving air and a given ambient temperature. For instance, air at [latex]0^\circ\text{C}[/latex] with a 15 m/s wind has the same chilling effect as still air at about [latex]-18^\circ\text{C}[/latex].
Interestingly, while air can transfer heat rapidly via convection, it is a poor thermal conductor. Whether air serves as an insulator or a conductor depends on the available space for it to circulate. In structures like homes, the cavity between inner and outer walls is often around 9 cm (3.5 inches), which is wide enough for convection currents to form. To reduce this unwanted heat loss, insulation is added to trap air in spaces too small to allow convection—making use of air’s low thermal conductivity.
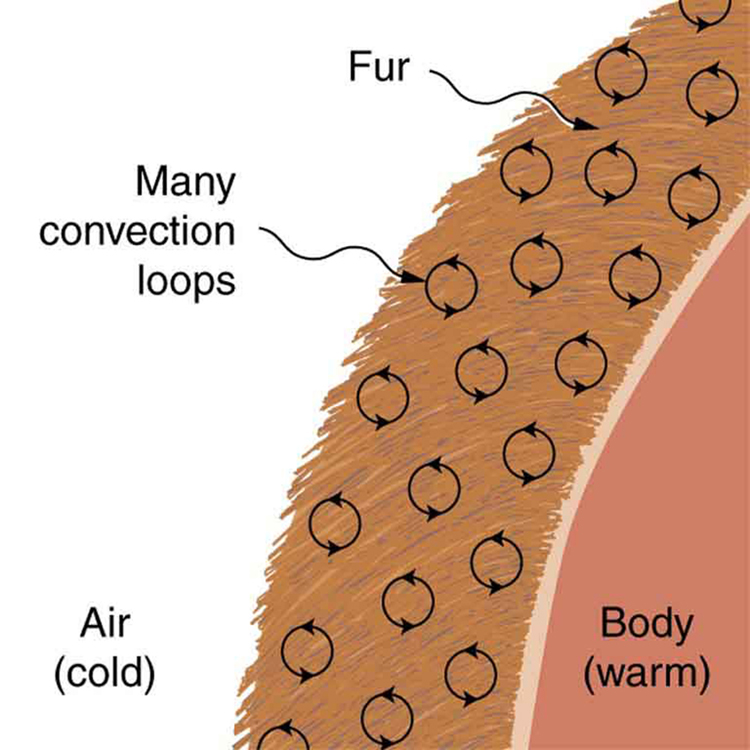
Windows often use the same principle. A double-pane window contains a narrow air gap of about 1 cm between the panes. This small space prevents convection from occurring while still benefiting from air’s poor heat conduction, minimizing energy loss. Fur, feathers, fiber, and fiberglass also operate on this principle. They trap air in very small pockets, preventing the formation of convective loops. This makes them excellent lightweight insulators for both animals and buildings.
One biologically significant phenomenon involves convection combined with a phase change. This is how sweating cools the body, even if the ambient air temperature is higher than body temperature. The phase change from liquid sweat to water vapor requires energy—specifically, heat from the skin. This latent heat of vaporization draws thermal energy away from the body. However, if the surrounding air becomes saturated with water vapor (humid conditions), evaporation stops. Air flow caused by convection removes this humid air and replaces it with drier air, allowing evaporation—and thus cooling—to continue.
Example 105.2: Calculate the Flow of Mass during Convection: Sweat-Heat Transfer away from the Body
The average person produces heat at the rate of about 120 W when at rest. At what rate must water evaporate from the body to get rid of all this energy? (This evaporation might occur when a person is sitting in the shade and surrounding temperatures are the same as skin temperature, eliminating heat transfer by other methods.)
Strategy
Energy is needed for a phase change ([latex]Q={\text{mL}}_{\text{v}}[/latex]). Thus, the energy loss per unit time is
We divide both sides of the equation by [latex]{L}_{\text{v}}[/latex] to find that the mass evaporated per unit time is
Solution
Insert the value of the latent heat from Table 102.1, [latex]{L}_{\text{v}}=\text{2430}\text{ kJ/kg}=\text{2430}\text{ J/g}[/latex]. This yields
Discussion
Evaporating about 3 g/min seems reasonable. This would be about 180 g (about 7 oz) per hour. If the air is very dry, the sweat may evaporate without even being noticed. A significant amount of evaporation also takes place in the lungs and breathing passages.
Another important real-world example of heat transfer by convection combined with a phase change occurs in Earth’s atmosphere—especially in weather systems. When ocean water evaporates, it removes heat from the surface. This water vapor is carried upward by convection. As it rises and cools, it condenses into tiny liquid droplets to form clouds. During this condensation, the latent heat that was absorbed during evaporation is now released into the atmosphere.
This release of energy heats the surrounding air, causing it to expand and rise further. As the warm air continues to ascend, it cools more, which causes additional condensation and releases even more heat. This is a classic example of a positive feedback loop: each step reinforces and accelerates the next. This process is what powers the rapid vertical growth of cumulus clouds, which can rise as high as 20 kilometers into the stratosphere. These towering thunderclouds are not only visually striking, but they can also produce lightning, hail, and hurricanes—all driven by the transfer of heat through convection and phase change.


Another example of convection combined with phase change is seen in the movement and melting of icebergs. As an iceberg formed in cold regions like Greenland drifts into warmer Atlantic waters, it melts. This melting process absorbs heat from the surrounding ocean. Conversely, when water freezes to form an iceberg, it releases heat into the surrounding environment. In both cases, the transfer of thermal energy is facilitated by the convection of ocean water, which circulates the energy throughout the system.

Check Your Understanding
Why does using a fan in the summer feel refreshing?
A fan increases air flow over your body, which enhances convection. The warm air layer around your skin is quickly replaced with cooler air, increasing the rate of heat loss from your body. This is why moving air “feels” cooler than still air—even if both are at the same temperature.
Summary
- Convection is the transfer of heat through the bulk movement of fluid (liquid or gas). It can occur naturally (due to buoyancy) or be forced (e.g., by a fan or pump).
- Convection often transfers thermal energy more efficiently than conduction and is especially powerful when accompanied by phase changes.
- Phase change convection occurs in biological processes (e.g., sweating), weather systems (e.g., cloud formation), and geophysical processes (e.g., iceberg melting). These systems often involve large energy exchanges.
Conceptual Questions
- One way to make a fireplace more energy efficient is to have an external air supply for the combustion of its fuel. Another is to have room air circulate around the outside of the fire box and back into the room. Detail the methods of heat transfer involved in each.
- On cold, clear nights horses will sleep under the cover of large trees. How does this help them keep warm?
Problems & Exercises
- At what wind speed does [latex]-\text{10º}\text{C}[/latex] air cause the same chill factor as still air at [latex]-\text{29º}\text{C}[/latex]?
- At what temperature does still air cause the same chill factor as [latex]-5º\text{C}[/latex] air moving at 15 m/s?
- The “steam” above a freshly made cup of instant coffee is really water vapor droplets condensing after evaporating from the hot coffee. What is the final temperature of 250 g of hot coffee initially at [latex]\text{90}\text{.}\text{0ºC}[/latex] if 2.00 g evaporates from it? The coffee is in a Styrofoam cup, so other methods of heat transfer can be neglected.
- (a) How many kilograms of water must evaporate from a 60.0-kg woman to lower her body temperature by [latex]0\text{.}\text{750ºC}[/latex]? (b) Is this a reasonable amount of water to evaporate in the form of perspiration, assuming the relative humidity of the surrounding air is low?
- On a hot dry day, evaporation from a lake has just enough heat transfer to balance the [latex]1\text{.}\text{00}{\text{ kW/m}}^{2}[/latex] of incoming heat from the Sun. What mass of water evaporates in 1.00 h from each square meter?
- One winter day, the climate control system of a large university classroom building malfunctions. As a result, [latex]\text{500 }{\text{m}}^{3}[/latex] of excess cold air is brought in each minute. At what rate in kilowatts must heat transfer occur to warm this air by [latex]\text{10}\text{.}\text{0ºC}[/latex] (that is, to bring the air to room temperature)?
- The Kilauea volcano in Hawaii is the world’s most active, disgorging about [latex]\text{5}×{\text{10}}^{5}\phantom{\rule{0.25em}{0ex}}{\text{m}}^{3}[/latex] of [latex]\text{1200ºC}[/latex] lava per day. What is the rate of heat transfer out of Earth by convection if this lava has a density of [latex]\text{2700}\phantom{\rule{0.25em}{0ex}}{\text{kg/m}}^{3}[/latex] and eventually cools to [latex]\text{30ºC}[/latex]? Assume that the specific heat of lava is the same as that of granite.

Figure 105.7: Lava flow on Kilauea volcano in Hawaii. (credit: J. P. Eaton, U.S. Geological Survey) - During heavy exercise, the body pumps 2.00 L of blood per minute to the surface, where it is cooled by [latex]2\text{.}\text{00ºC}[/latex]. What is the rate of heat transfer from this forced convection alone, assuming blood has the same specific heat as water and its density is [latex]\text{1050}{\text{ kg/m}}^{3}[/latex]?
- A person inhales and exhales 2.00 L of [latex]\text{37}\text{.}\text{0ºC}[/latex] air, evaporating [latex]4\text{.}\text{00}×{\text{10}}^{-2}\text{ g}[/latex] of water from the lungs and breathing passages with each breath. (a) How much heat transfer occurs due to evaporation in each breath? (b) What is the rate of heat transfer in watts if the person is breathing at a moderate rate of 18.0 breaths per minute? (c) If the inhaled air had a temperature of [latex]\text{20}\text{.}0º\text{C}[/latex], what is the rate of heat transfer for warming the air? (d) Discuss the total rate of heat transfer as it relates to typical metabolic rates. Will this breathing be a major form of heat transfer for this person?
- A glass coffee pot has a circular bottom with a 9.00-cm diameter in contact with a heating element that keeps the coffee warm with a continuous heat transfer rate of 50.0 W (a) What is the temperature of the bottom of the pot, if it is 3.00 mm thick and the inside temperature is [latex]\text{60}\text{.}\text{0ºC}[/latex]? (b) If the temperature of the coffee remains constant and all of the heat transfer is removed by evaporation, how many grams per minute evaporate? Take the heat of vaporization to be 2340 kJ/kg.

