Heat and Heat Transfer Methods
104 Conduction
Learning Objectives
- Describe how thermal conduction occurs at the molecular level.
- Calculate the rate of heat transfer using the thermal conductivity equation.
- Compare the thermal conductivities of common materials.

Imagine walking barefoot across your carpeted living room floor and then stepping onto a tile kitchen floor. Both surfaces are at the same temperature, yet the tile feels colder. Why? The key lies in how quickly each material transfers heat. The tile conducts heat away from your skin faster than the carpet does, creating a sharper drop in skin temperature and a stronger sensation of cold.
Different materials transfer heat at different rates. In general, metals—like copper, aluminum, and silver—are excellent thermal conductors. In contrast, materials like rubber, wood, and plastic are thermal insulators. The underlying reason lies in how molecules interact. As shown in Figure 104.2, molecules in hotter materials have more kinetic energy than those in colder materials. When they collide at a boundary, energy is transferred from the hotter to the cooler side. This microscopic exchange forms the basis of conduction.
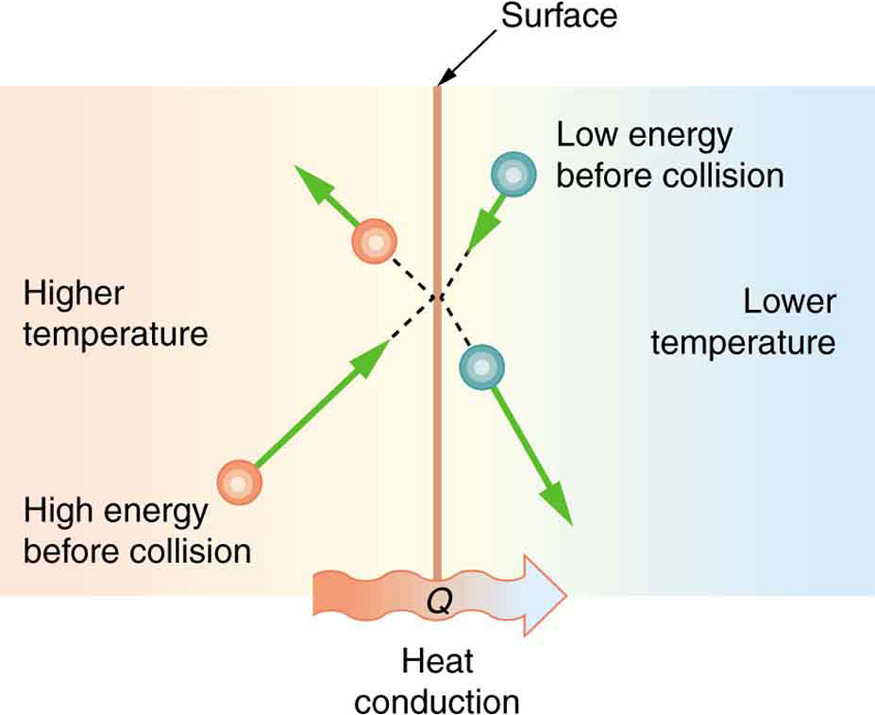
Several factors affect how much heat is transferred by conduction:
- Temperature difference: A larger difference between hot and cold regions increases the rate of heat transfer.
- Contact area: A greater surface area allows more molecules to collide and transfer energy.
- Material thickness: Thicker barriers slow down heat transfer, which is why layers of fat or insulation help retain body heat or indoor warmth.
- Thermal conductivity: Each material has a specific ability to conduct heat, measured by a constant known as thermal conductivity ([latex]k[/latex]).
These factors are combined in the following formula for the rate of heat transfer by conduction:
Where:
- [latex]\frac{Q}{t}[/latex] is the rate of heat transfer (watts or kcal/s)
- [latex]k[/latex] is the thermal conductivity of the material
- [latex]A[/latex] is the cross-sectional area
- [latex]T_2 - T_1[/latex] is the temperature difference across the material
- [latex]d[/latex] is the material thickness
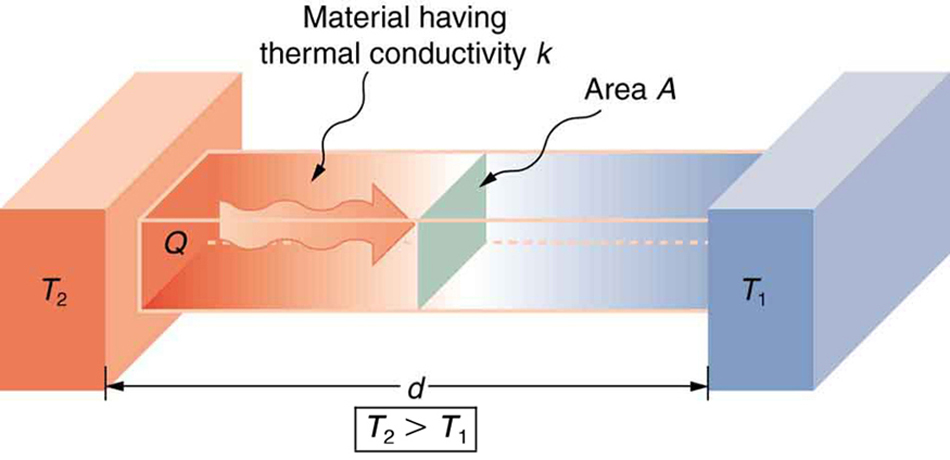
This equation lets us quantify how heat moves through biological tissues (like skin and fat), building materials (like wood or insulation), or even protective layers like fur and blubber in animals. For health science applications, this is essential when analyzing hypothermia risk, thermal burns, or patient warming methods (see Figure 104.3).
Thermal conductivity values for common materials are given in Table 104.1 (provided in the next section). These help predict and compare how different substances behave in various heat-related situations.
Example 104.1 Calculating Heat Transfer Through Conduction: Conduction Rate Through an Ice Box
A Styrofoam ice box has a total area of [latex]0\text{.950}\phantom{\rule{0.25em}{0ex}}{\text{ m}}^{2}[/latex] and walls with an average thickness of 2.50 cm. The box contains ice, water, and canned beverages at [latex]\text{0ºC}[/latex]. The inside of the box is kept cold by melting ice. How much ice melts in one day if the ice box is kept in the trunk of a car at [latex]\text{35}\text{.}\text{0ºC}[/latex]?
Strategy
This question involves both heat for a phase change (melting of ice) and the transfer of heat by conduction. To find the amount of ice melted, we must find the net heat transferred. This value can be obtained by calculating the rate of heat transfer by conduction and multiplying by time.
Solution
- Identify the knowns.
[latex]A=0\text{.}\text{950}{\text{ m}}^{2}\mathrm{; }[/latex]
[latex]d=2\text{.}\text{50}\text{ cm}=0\text{.0250 m;}[/latex]
[latex]A\phantom{\rule{0.25em}{0ex}}{T}_{1}= 0º\text{C;}[/latex]
[latex]A\phantom{\rule{0.25em}{0ex}}{T}_{2}=\text{35}\text{.}0º\text{C,}\phantom{\rule{0.25em}{0ex}}t=\text{1 day}=\text{24 hours}=\text{86,400 s.}[/latex]
- Identify the unknowns. We need to solve for the mass of the ice, [latex]m[/latex]. We will also need to solve for the net heat transferred to melt the ice, [latex]Q[/latex].
- Determine which equations to use. The rate of heat transfer by conduction is given by
[latex]\frac{Q}{t}=\frac{\text{kA}\left({T}_{2}-{T}_{1}\right)}{d}\text{.}[/latex]
- The heat is used to melt the ice: [latex]Q={\text{mL}}_{\text{f}}.[/latex]
- Insert the known values:
[latex]\frac{Q}{t}=\frac{\left(\text{0.010 J/s}\cdot \text{m}\cdot º\text{C}\right)\left(\text{0.950}\phantom{\rule{0.25em}{0ex}}{\text{ m}}^{2}\right)\left(\text{35.}0º\text{C}-0º\text{C}\right)}{\text{0.0250 m}}=\text{13.3 J/s.}[/latex]
- Multiply the rate of heat transfer by the time ([latex]1\text{ day = 86,400}\text{ s}[/latex]):
[latex]Q=\left(Q/t\right)t=\left(\text{13}\text{.}3\text{ J/s}\right)\left(\text{86},\text{400}\text{ s}\right)=1\text{.}\text{15}×{\text{10}}^{6}\text{ J.}[/latex]
- Set this equal to the heat transferred to melt the ice: [latex]Q={\text{mL}}_{\text{f}}[/latex]. Solve for the mass [latex]m[/latex]:
[latex]m=\frac{Q}{{L}_{\text{f}}}=\frac{1\text{.}\text{15}×{\text{10}}^{6}\text{ J}}{\text{334 }×{\text{10}}^{3}\text{ J/kg}}=3\text{.}\text{44}\text{kg.}[/latex]
Discussion
The result of 3.44 kg, or about 7.6 lbs, seems about right, based on experience. You might expect to use about a 4 kg (7–10 lb) bag of ice per day. A little extra ice is required if you add any warm food or beverages.
Inspecting the conductivities in Table 104.1 shows that Styrofoam is a very poor conductor and thus a good insulator. Other good insulators include fiberglass, wool, and goose-down feathers. Like Styrofoam, these all incorporate many small pockets of air, taking advantage of air’s poor thermal conductivity.
| Substance | Thermal conductivity [latex]\mathbf{\text{k (J/s⋅m⋅ºC)}}[/latex] |
|---|---|
| Silver | 420 |
| Copper | 390 |
| Gold | 318 |
| Aluminum | 220 |
| Steel iron | 80 |
| Steel (stainless) | 14 |
| Ice | 2.2 |
| Glass (average) | 0.84 |
| Concrete brick | 0.84 |
| Water | 0.6 |
| Fatty tissue (without blood) | 0.2 |
| Asbestos | 0.16 |
| Plasterboard | 0.16 |
| Wood | 0.08–0.16 |
| Snow (dry) | 0.10 |
| Cork | 0.042 |
| Glass wool | 0.042 |
| Wool | 0.04 |
| Down feathers | 0.025 |
| Air | 0.023 |
| Styrofoam | 0.010 |
To design effective thermal insulators—whether for building construction, surgical blankets, or biomedical devices—we often combine material selection with appropriate thickness. In general, an ideal insulator should have a low thermal conductivity ([latex]k[/latex]) and a high thickness ([latex]d[/latex]). This relationship can be captured using the R factor, which is defined as:
The R factor (or R-value) is a measure of thermal resistance. It tells us how well a material resists heat flow by conduction. A higher [latex]R[/latex] value means better insulation, because the rate of heat transfer is inversely proportional to [latex]R[/latex]:
In practical terms, if we double the thickness of an insulating layer without changing the material, we double the [latex]R[/latex] value. If we use a material with a lower thermal conductivity, we also raise [latex]R[/latex]. This makes [latex]R[/latex] a convenient metric for selecting building materials or designing thermal protection systems in medical applications.
Although [latex]R[/latex] values are defined in SI units, in practice they are often quoted in non-metric units—especially in the U.S. building industry. Typical units are:
- [latex]\text{ft}^2 \cdot {}^\circ\text{F} \cdot \text{h} / \text{Btu}[/latex] (British thermal units)
Here are a few representative examples:
- [latex]R = 11[/latex] for fiberglass batts that are 3.5 inches thick (used in standard walls)
- [latex]R = 19[/latex] for fiberglass batts that are 6.5 inches thick (commonly used in ceilings)
In colder climates or in sensitive applications—such as neonatal incubators or cryogenic sample storage—greater thickness or better materials may be used to raise [latex]R[/latex] and thus reduce heat transfer further.
Note in Table 104.1 that materials with the highest thermal conductivity—like silver, copper, gold, and aluminum—are also excellent electrical conductors. This is because both heat and electricity in these materials are transported by free electrons. This dual property makes metals like copper and aluminum especially useful in cookware, electronics, and thermal exchange systems.
In contrast, insulators used in walls, lab freezers, or surgical warming blankets rely on poor electron mobility and minimal molecular vibration transfer—hence their low [latex]k[/latex] and high [latex]R[/latex] values.
Example 104.2 Calculating the Temperature Difference Maintained by a Heat Transfer: Conduction Through an Aluminum Pan
Water is boiling in an aluminum pan placed on an electrical element on a stovetop. The sauce pan has a bottom that is 0.800 cm thick and 14.0 cm in diameter. The boiling water is evaporating at the rate of 1.00 g/s. What is the temperature difference across (through) the bottom of the pan?
Strategy
Conduction through the aluminum is the primary method of heat transfer here, and so we use the equation for the rate of heat transfer and solve for the temperature difference.
Solution
- Identify the knowns and convert them to the SI units.
The thickness of the pan, [latex]d=0\text{.800 cm}=8.0×{\text{10}}^{-3}\phantom{\rule{0.25em}{0ex}}\text{ m,}[/latex] the area of the pan, [latex]A=\pi (0\text{.14}/2)^{2}\phantom{\rule{0.25em}{0ex}}{\text{ m}}^{2}=1\text{.}\text{54}×{\text{10}}^{-2}\phantom{\rule{0.25em}{0ex}}{\text{ m}}^{2}[/latex], and the thermal conductivity, [latex]k=\text{220 J/s}\cdot m\cdot °C.[/latex]
- Calculate the necessary heat of vaporization of 1 g of water:
[latex]Q={\text{mL}}_{\text{v}}=\left(1\text{.}00×{\text{10}}^{-3}\text{ kg}\right)\left(\text{2256}×{\text{10}}^{3}\text{ J/kg}\right)=\text{2256}\mathrm{ J.}[/latex]
- Calculate the rate of heat transfer given that 1 g of water melts in one second:
[latex]Q/t=\text{2256}\text{ J/s or 2.26 kW.}[/latex]
- Insert the knowns into the equation and solve for the temperature difference:
[latex]{T}_{2}-{T}_{1}=\frac{Q}{t}\left(\frac{d}{\text{kA}}\right)=\left(\text{2256}\text{ J/s}\right)\frac{8\text{.}\text{00} × {\text{10}}^{-3}\text{m}}{\left(\text{220}\text{ J/s}\cdot \text{m}\cdot º\text{C}\right)\left(1\text{.}\text{54}×{\text{10}}^{-2}{\text{ m}}^{2}\right)}=5\text{.}33º\mathrm{C.}[/latex]
Discussion
The value for the heat transfer [latex]Q/t\text{ = 2}\text{.}\text{26}\text{kW}\text{ or }\text{2256}\text{ J/s}[/latex] is typical for an electric stove. This value gives a remarkably small temperature difference between the stove and the pan. Consider that the stove burner is red hot while the inside of the pan is nearly [latex]\text{100ºC}[/latex] because of its contact with boiling water. This contact effectively cools the bottom of the pan in spite of its proximity to the very hot stove burner. Aluminum is such a good conductor that it only takes this small temperature difference to produce a heat transfer of 2.26 kW into the pan.
Conduction is caused by the random motion of atoms and molecules. As such, it is an ineffective mechanism for heat transport over macroscopic distances and short time distances. Take, for example, the temperature on the Earth, which would be unbearably cold during the night and extremely hot during the day if heat transport in the atmosphere was to be only through conduction. In another example, car engines would overheat unless there was a more efficient way to remove excess heat from the pistons.
Check Your Understanding
How does the rate of heat transfer by conduction change when all spatial dimensions (e.g., thickness and surface area) of an object are doubled?
When all linear dimensions of an object are doubled, its surface area increases by a factor of four because area depends on the square of the length:
Meanwhile, the thickness (or distance for conduction) doubles:
The temperature difference and the thermal conductivity remain unchanged. Using the equation for conductive heat transfer, we find:
Conclusion: The rate of heat transfer doubles. This is because the increase in surface area (×4) outweighs the increase in thickness (×2), leading to an overall factor of 2 increase in heat transfer rate.
Summary
- Conduction is the transfer of heat between substances that are in direct contact.
- The rate of heat transfer by conduction depends on material properties and geometry. It increases with:
- greater thermal conductivity [latex]k[/latex],
- larger contact area [latex]A[/latex], and
- larger temperature difference [latex]T_2 - T_1[/latex].
- It decreases with increased thickness [latex]d[/latex] of the material.
- The equation for the rate of heat transfer by conduction is:
This relationship is essential for understanding biological systems (e.g., thermal regulation in skin or tissue layers) and for designing medical devices and protective gear.
Conceptual Questions
- Some electric stoves have a flat ceramic surface with heating elements hidden beneath. A pot placed over a heating element will be heated, while it is safe to touch the surface only a few centimeters away. Why is ceramic, with a conductivity less than that of a metal but greater than that of a good insulator, an ideal choice for the stove top?
- Loose-fitting white clothing covering most of the body is ideal for desert dwellers, both in the hot Sun and during cold evenings. Explain how such clothing is advantageous during both day and night

Figure 104.5 A jellabiya is worn by many men in Egypt. (credit: Zerida)
Problems & Exercises
- (a) Calculate the rate of heat conduction through house walls that are 13.0 cm thick and that have an average thermal conductivity twice that of glass wool. Assume there are no windows or doors. The surface area of the walls is [latex]\text{120}\phantom{\rule{0.25em}{0ex}}{\text{m}}^{2}[/latex] and their inside surface is at [latex]\text{18.}0º\text{C}[/latex], while their outside surface is at [latex]5\text{.00º}\text{C}[/latex]. (b) How many 1-kW room heaters would be needed to balance the heat transfer due to conduction?
- The rate of heat conduction out of a window on a winter day is rapid enough to chill the air next to it. To see just how rapidly the windows transfer heat by conduction, calculate the rate of conduction in watts through a [latex]3\text{.}{\text{00-m}}^{2}[/latex] window that is [latex]0\text{.635 cm}[/latex] thick (1/4 in) if the temperatures of the inner and outer surfaces are [latex]5\text{.00ºC}[/latex] and [latex]-\text{10}\text{.}0º\text{C}[/latex], respectively. This rapid rate will not be maintained—the inner surface will cool, and even result in frost formation.
- Calculate the rate of heat conduction out of the human body, assuming that the core internal temperature is [latex]\text{37}\text{.}0º\text{C}[/latex], the skin temperature is [latex]\text{34}\text{.}0º\text{C}[/latex], the thickness of the tissues between averages [latex]1\text{.00 cm}[/latex], and the surface area is [latex]1\text{.}\text{40}\phantom{\rule{0.25em}{0ex}}{\text{m}}^{2}[/latex].
- Suppose you stand with one foot on ceramic flooring and one foot on a wool carpet, making contact over an area of [latex]\text{80}\text{.}0\phantom{\rule{0.25em}{0ex}}{\text{cm}}^{2}[/latex] with each foot. Both the ceramic and the carpet are 2.00 cm thick and are [latex]\text{10.}0º\text{C}[/latex] on their bottom sides. At what rate must heat transfer occur from each foot to keep the top of the ceramic and carpet at [latex]\text{33}\text{.}0º\text{C}[/latex]?
- A man consumes 3000 kcal of food in one day, converting most of it to maintain body temperature. If he loses half this energy by evaporating water (through breathing and sweating), how many kilograms of water evaporate?
- (a) A firewalker runs across a bed of hot coals without sustaining burns. Calculate the heat transferred by conduction into the sole of one foot of a firewalker given that the bottom of the foot is a 3.00-mm-thick callus with a conductivity at the low end of the range for wood and its density is [latex]\text{300}{\text{ kg/m}}^{3}[/latex]. The area of contact is [latex]\text{25}\text{.}0{\text{ cm}}^{2}[/latex], the temperature of the coals is [latex]\text{700º}\text{C}[/latex], and the time in contact is 1.00 s. (b) What temperature increase is produced in the [latex]\text{25}\text{.}0{\text{ cm}}^{3}[/latex] of tissue affected? (c) What effect do you think this will have on the tissue, keeping in mind that a callus is made of dead cells?
- (a) What is the rate of heat conduction through the 3.00-cm-thick fur of a large animal having a [latex]1\text{.}{\text{40-m}}^{2}[/latex] surface area? Assume that the animal’s skin temperature is [latex]\text{32}\text{.}0º\text{C}[/latex], that the air temperature is [latex]-5\text{.}\text{00º}\text{C}[/latex], and that fur has the same thermal conductivity as air. (b) What food intake will the animal need in one day to replace this heat transfer?
- A walrus transfers energy by conduction through its blubber at the rate of 150 W when immersed in [latex]-1\text{.00ºC}[/latex] water. The walrus’s internal core temperature is [latex]\text{37.}0º\text{C}[/latex], and it has a surface area of [latex]2\text{.00}\phantom{\rule{0.25em}{0ex}}{\text{m}}^{2}[/latex]. What is the average thickness of its blubber, which has the conductivity of fatty tissues without blood?
- Compare the rate of heat conduction through a 13.0-cm-thick wall that has an area of [latex]\text{10}\text{.}0{\text{ m}}^{2}[/latex] and a thermal conductivity twice that of glass wool with the rate of heat conduction through a window that is 0.750 cm thick and that has an area of [latex]2\text{.}\text{00}{\text{ m}}^{2}[/latex], assuming the same temperature difference across each.
- Suppose a person is covered head to foot by wool clothing with average thickness of 2.00 cm and is transferring energy by conduction through the clothing at the rate of 50.0 W. What is the temperature difference across the clothing, given the surface area is [latex]1\text{.}\text{40}{\text{ m}}^{2}[/latex]?
- Some stove tops are smooth ceramic for easy cleaning. If the ceramic is 0.600 cm thick and heat conduction occurs through the same area and at the same rate as computed in Example 104.2, what is the temperature difference across it? Ceramic has the same thermal conductivity as glass and brick.
- One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a house. Suppose the house already had 15 cm of fiberglass insulation in the attic and in all the exterior surfaces. If you added an extra 8.0 cm of fiberglass to the attic, then by what percentage would the heating cost of the house drop? Take the single story house to be of dimensions 10 m by 15 m by 3.0 m. Ignore air infiltration and heat loss through windows and doors.
- (a) Calculate the rate of heat conduction through a double-paned window that has a [latex]1\text{.}\text{50}{\text{-m}}^{2}[/latex] area and is made of two panes of 0.800-cm-thick glass separated by a 1.00-cm air gap. The inside surface temperature is [latex]\text{15}\text{.}0º\text{C}[/latex], while that on the outside is [latex]-\text{10}\text{.}0º\text{C}[/latex]. (Hint: There are identical temperature drops across the two glass panes. First find these and then the temperature drop across the air gap. This problem ignores the increased heat transfer in the air gap due to convection.) (b) Calculate the rate of heat conduction through a 1.60-cm-thick window of the same area and with the same temperatures. Compare your answer with that for part (a).
- Many decisions are made on the basis of the payback period: the time it will take through savings to equal the capital cost of an investment. Acceptable payback times depend upon the business or philosophy one has. (For some industries, a payback period is as small as two years.) Suppose you wish to install the extra insulation in exercise 12 above. If energy cost $1.00 per million joules and the insulation was ?4.00 per square meter, then calculate the simple payback time. Take the average [latex]\text{Δ}T[/latex] for the 120 day heating season to be [latex]\text{15.}0º\text{C}[/latex].
- For the human body, what is the rate of heat transfer by conduction through the body’s tissue with the following conditions: the tissue thickness is 3.00 cm, the change in temperature is [latex]2\text{.}\text{00º}\text{C}[/latex], and the skin area is [latex]1\text{.}\text{50}\phantom{\rule{0.25em}{0ex}}{\text{ m}}^{2}[/latex]. How does this compare with the average heat transfer rate to the body resulting from an energy intake of about 2400 kcal per day? (No exercise is included.)
Footnotes
- 1 At temperatures near 0ºC.
Glossary
- R factor
- the ratio of thickness to the conductivity of a material
- rate of conductive heat transfer
- rate of heat transfer from one material to another
- thermal conductivity
- the property of a material’s ability to conduct heat
the spontaneous transfer of energy due to a temperature difference
the energy an object has by reason of its motion, equal to [latex]\frac{1}{2}{\text{mv}}^{2}[/latex] for the translational (i.e., non-rotational) motion of an object of mass [latex]m[/latex] moving at speed [latex]v[/latex]
the ratio of thickness to the conductivity of a material
rate of heat transfer from one material to another
the property of a material’s ability to conduct heat


