Introduction: The Nature of Science and Physics
4 Accuracy, Precision, and Significant Figures
Learning Objectives
- Determine the appropriate number of significant figures in both addition and subtraction, as well as multiplication and division calculations.
- Calculate the percent uncertainty of a measurement.

Accuracy and Precision of a Measurement
Biologists, ecologists, geneticists, and other life scientists all rely on measurements to make sense of the natural world. Whether you’re measuring the size of a cell under a microscope, the growth rate of plants in an experiment, or the migration speed of an animal tracked by GPS, the quality of your data depends on how accurate and precise your measurements are.
-
Accuracy refers to how close a measured value is to the true or accepted value.
-
Precision refers to how close repeated measurements are to one another, regardless of how close they are to the true value.
Let’s take a simple example: measuring the length of a leaf. Suppose your measuring tool is a digital caliper that gives values to the nearest 0.1 mm. You measure the same leaf three times and get: 84.1 mm, 84.2 mm, and 84.0 mm. These results are both accurate (if the actual length is about 84.1 mm) and precise (since the values are very close together).
Now imagine instead you record 82.0 mm, 92.3 mm, and 76.9 mm. These values vary a lot—low precision. Or you measure 79.5 mm three times, which is very consistent (high precision) but not close to the actual length (low accuracy).
Accuracy vs. Precision: Visualizing the Difference
Imagine you’re using a GPS collar to track the position of an endangered bird nesting in a wildlife preserve like in Figure 4.3, andFigure 4.4,.
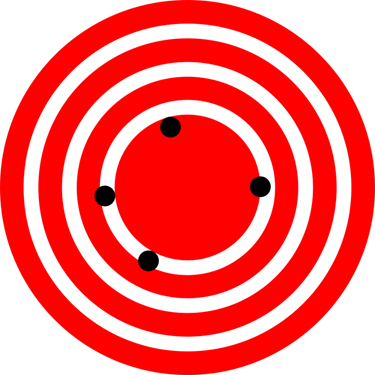
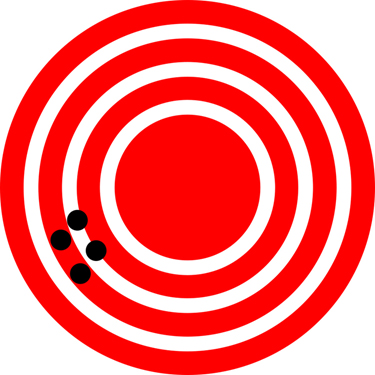
Both cases can be problematic depending on the context. In science, we aim for measurements that are both accurate and precise.
Uncertainty in Measurements
Every measurement includes some amount of uncertainty, which is a numerical estimate of how much a measured value could differ from the true value.
For example, if you weigh a small sample of algae and the mass reads 2.50 g ± 0.02 g, this means the true mass is probably between 2.48 g and 2.52 g. The “±” value is the absolute uncertainty.
Uncertainty arises from:
-
Limitations of the measuring device (e.g., ruler marked only in mm)
-
User technique (e.g., misreading or parallax error)
-
Object irregularities (e.g., wrinkled or asymmetrical leaves)
-
Environmental conditions (e.g., temperature, humidity)
Uncertainty is often written as:
A ± δA
where A is the measured value, and δA (delta A) is the uncertainty.
Example: A biologist measures a fish’s length as 12.0 ± 0.5 cm, meaning the real length is likely between 11.5 and 12.5 cm.
Real-World Connection: Is That a Fever?
Imagine you’re measuring body temperature using a thermometer. If the reading is 37.0°C (normal) but the uncertainty is ±3.0°C, then the person’s real temperature could be anywhere from 34.0°C (hypothermic) to 40.0°C (fever). That range is far too large to be useful. Accurate and precise thermometers are essential in both clinical and laboratory environments.
This same principle applies in ecology, genetics, and microbiology—whether you’re tracking insect populations or measuring pH levels in a solution, you must understand how confident you are in your measurements.
Percent Uncertainty
To compare uncertainty across measurements of different sizes, we often express uncertainty as a percentage. This is called percent uncertainty:
Where:
-
A is the measured value,
-
δA is the absolute uncertainty.
Example: If a DNA sample weighs 2.00 g with an uncertainty of 0.05 g, the percent uncertainty is:
Percent uncertainty helps you determine how much trust to place in your measurements—and helps others evaluate your data, especially in peer-reviewed research or clinical studies.
Example 4.1: Calculating Percent Uncertainty: A Bag of Apples
A grocery store sells [latex]\text{5-lb}[/latex] bags of apples. You purchase four bags over the course of a month and weigh the apples each time. You obtain the following measurements:
- Week 1 weight: [latex]\text{4.8 lb}[/latex]
- Week 2 weight: [latex]\text{5.3 lb}[/latex]
- Week 3 weight: [latex]\text{4.9 lb}[/latex]
- Week 4 weight: [latex]\text{5.4 lb}[/latex]
You determine that the weight of the [latex]\text{5-lb}[/latex] bag has an uncertainty of [latex]±0\text{.}4\phantom{\rule{0.25em}{0ex}}\text{lb}[/latex]. What is the percent uncertainty of the bag’s weight?
Strategy
First, observe that the expected value of the bag’s weight, [latex]A[/latex], is 5 lb. The uncertainty in this value, [latex]\mathrm{\delta A}[/latex], is 0.4 lb. We can use the following equation to determine the percent uncertainty of the weight:
Solution
Plug the known values into the equation:
Discussion
We can conclude that the weight of the apple bag is [latex]5\phantom{\rule{0.25em}{0ex}}\text{lb}±8\text{%}[/latex]. Consider how this percent uncertainty would change if the bag of apples were half as heavy, but the uncertainty in the weight remained the same. Hint for future calculations: when calculating percent uncertainty, always remember that you must multiply the fraction by 100%. If you do not do this, you will have a decimal quantity, not a percent value.
Uncertainty in Calculated Results
When we make measurements and then use them in calculations—like area, volume, or reaction rates—those calculations also carry uncertainty. In biology and life sciences, this applies when calculating population densities, nutrient concentrations, enzyme reaction rates, or even field plot areas.
Let’s say you’re calculating the area of a rectangular leaf based on its measured length and width. Both measurements carry uncertainty, so your calculated area will too.
If your values have small uncertainties (typically a few percent or less), you can use the method of adding percent uncertainties:
For multiplication or division:
The percent uncertainty of the final result is the sum of the percent uncertainties in the measurements used.
Example: Calculating Leaf Surface Area
Suppose you measure a leaf as:
-
Length = 4.00 cm with a 2% uncertainty
-
Width = 3.00 cm with a 1% uncertainty
The area is:
The total percent uncertainty is:
So the uncertainty in area is:
Final result:
Check Your Understanding
A field biologist measures the time it takes two tagged turtles to cross a 10-meter stream. The stopwatch has an uncertainty of ±0.05 s. Turtle A finishes in 12.04 s, and Turtle B finishes in 12.07 s.
Can the stopwatch reliably tell which turtle was faster?
Answer: No—since the time difference (0.03 s) is smaller than the uncertainty (±0.05 s), the stopwatch is not precise enough to distinguish the two.
Precision of Measuring Tools and Significant Figures
Different measuring tools offer different levels of precision, meaning they can measure smaller increments.
-
A ruler might measure to the nearest millimeter.
-
A digital caliper might measure to the nearest 0.01 mm.
-
A micropipette might dispense liquid in microliters (μL).
When recording a measurement, you can only report digits your tool allows you to measure, plus one estimated digit.
Example: If a microscope reticle lets you measure to the nearest 0.1 mm, and a bacterial colony falls between 2.2 mm and 2.3 mm, you might estimate the size as 2.25 mm. The “5” is an estimated digit.
Significant Figures (Sig Figs)
Significant figures are the digits in a measurement that carry meaning about its precision.
-
All non-zero digits are significant.
-
Zeros between significant digits are significant.
-
Leading zeros are not significant (e.g., 0.0053 has 2 sig figs).
-
Trailing zeros in a number with a decimal point are significant (e.g., 15.4500 has 6 sig figs).
-
For ambiguous cases like 1300, use scientific notation (e.g., 1.30 × 10³ means 3 sig figs).
Example: 36.7 cm has 3 sig figs, while 0.00090 has 2 sig figs.
Significant Figures in Calculations
Multiplication/Division Rule:
-
The result has the same number of significant figures as the measurement with the fewest sig figs.
Example:
Area of a petri dish:
Addition/Subtraction Rule:
-
The result should have the same number of decimal places as the value with the fewest decimal places.
Example:
In This Textbook
To keep calculations clear and relevant:
-
Most values use three significant figures, unless context demands more.
-
Final answers reflect the least precise input value.
-
Exact numbers (like “2” in
) do not affect significant figure rules.
Check Your Understanding
Q1: A researcher carries three samples of leaf litter: two weigh 13.5 g, and one weighs 10.2 g. What is the total mass?
A1: 13.5 + 13.5 + 10.2 = 37.2 g (Exact counts of items don’t affect sig figs.)
Q2: A lab slide accelerates on a tilted stage at 0.0255 m/s². If its mass is 55 kg, what is the net force?
Summary
-
-
Accuracy describes how close a measured value is to the true or accepted value. In biological experiments, this ensures your measurements reflect real phenomena—such as actual enzyme concentration or body temperature.
-
Uncertainty estimates how much a measured value might differ from the true value. All measurements include some level of uncertainty due to limitations in tools, human error, or environmental factors.
-
Precision refers to how consistently repeated measurements agree with one another. For example, measuring the same sample of DNA concentration multiple times with similar results indicates high precision.
-
The precision of a tool depends on how finely it can measure. Instruments with smaller measurement increments (e.g., microliters or micrometers) are more precise and allow more accurate biological analysis.
-
Significant figures (sig figs) indicate the level of precision in a measurement and reflect the reliability of the measuring tool used.
-
When multiplying or dividing measurements, your final result should have the same number of significant figures as the value with the fewest sig figs.
-
When adding or subtracting, the answer should be rounded to match the least number of decimal places from the measured values.
-
Conceptual Questions
- What is the relationship between the accuracy and uncertainty of a measurement?
- Prescriptions for vision correction are given in units called diopters (D). Determine the meaning of that unit. Obtain information (perhaps by calling an optometrist or performing an internet search) on the minimum uncertainty with which corrections in diopters are determined and the accuracy with which corrective lenses can be produced. Discuss the sources of uncertainties in both the prescription and accuracy in the manufacture of lenses.
Problems & Exercises
Express your answers to problems in this section to the correct number of significant figures and proper units.
- Suppose that your bathroom scale reads your mass as 65 kg with a 3% uncertainty. What is the uncertainty in your mass (in kilograms)?
- A good-quality measuring tape can be off by 0.50 cm over a distance of 20 m. What is its percent uncertainty?
- Answer the following questions
- A car speedometer has a [latex]5.0\text{%}[/latex] uncertainty. What is the range of possible speeds when it reads [latex]\text{90}\phantom{\rule{0.25em}{0ex}}\text{km/h}[/latex]?
- Convert this range to miles per hour. [latex]\left(\text{1 km}=\text{0.6214 mi}\right)[/latex]
- An infant’s pulse rate is measured to be [latex]\text{130}±5[/latex] beats/min. What is the percent uncertainty in this measurement?
- Answer the following questions
- Suppose that a person has an average heart rate of 72.0 beats/min. How many beats does he or she have in 2.0 y?
- In 2.00 y?
- In 2.000 y?
- A can contains 375 mL of soda. How much is left after 308 mL is removed?
- State how many significant figures are proper in the results of the following calculations:
- [latex]\left(\text{106}\text{.}7\right)\left(\text{98}\text{.}2\right)/\left(\text{46}\text{.}\text{210}\right)\left(1\text{.}\text{01}\right)[/latex]
- [latex]{\left(\text{18}\text{.}7\right)}^{2}[/latex]
- [latex]\left(1\text{.}\text{60}×{\text{10}}^{-\text{19}}\right)\left(\text{3712}\right)[/latex].
- Answer the following questions
- How many significant figures are in the numbers 99 and 100?
- If the uncertainty in each number is 1, what is the percent uncertainty in each?
- Which is a more meaningful way to express the accuracy of these two numbers, significant figures or percent uncertainties?
- Answer the following questions
- If your speedometer has an uncertainty of [latex]2\text{.}0\phantom{\rule{0.25em}{0ex}}\text{km/h}[/latex] at a speed of [latex]\text{90}\phantom{\rule{0.25em}{0ex}}\text{km/h}[/latex], what is the percent uncertainty?
- If it has the same percent uncertainty when it reads [latex]\text{60}\phantom{\rule{0.25em}{0ex}}\text{km/h}[/latex], what is the range of speeds you could be going?
- Answer the following questions
- A person’s blood pressure is measured to be [latex]\text{120}±2\phantom{\rule{0.25em}{0ex}}\text{mm Hg}[/latex]. What is its percent uncertainty?
- Assuming the same percent uncertainty, what is the uncertainty in a blood pressure measurement of [latex]\text{80}\phantom{\rule{0.25em}{0ex}}\text{mm Hg}[/latex]?
- A person measures his or her heart rate by counting the number of beats in [latex]\text{30}\phantom{\rule{0.25em}{0ex}}\text{s}[/latex]. If [latex]\text{40}±1[/latex] beats are counted in [latex]\text{30}\text{.}0±0\text{.}5\phantom{\rule{0.25em}{0ex}}\text{s}[/latex], what is the heart rate and its uncertainty in beats per minute?
- What is the area of a circle [latex]3\text{.}\text{102}\phantom{\rule{0.25em}{0ex}}\text{cm}[/latex] in diameter?
- If a marathon runner averages 9.5 mi/h, how long does it take him or her to run a 26.22-mi marathon?
- A marathon runner completes a [latex]\text{42}\text{.}\text{188}\text{-km}[/latex] course in [latex]2\phantom{\rule{0.25em}{0ex}}\text{h}[/latex], 30 min, and [latex]\text{12}\phantom{\rule{0.25em}{0ex}}\text{s}[/latex]. There is an uncertainty of [latex]\text{25}\phantom{\rule{0.25em}{0ex}}\text{m}[/latex] in the distance traveled and an uncertainty of 1 s in the elapsed time.
- Calculate the percent uncertainty in the distance.
- Calculate the uncertainty in the elapsed time.
- What is the average speed in meters per second?
- What is the uncertainty in the average speed?
- The sides of a small rectangular box are measured to be [latex]1\text{.}\text{80}±0\text{.}\text{01}\phantom{\rule{0.25em}{0ex}}\text{cm}[/latex], [latex][/latex][latex]2\text{.}\text{05}±0\text{.}\text{02}\phantom{\rule{0.25em}{0ex}}\text{cm, and 3}\text{.}1±0\text{.}\text{1 cm}[/latex] long. Calculate its volume and uncertainty in cubic centimeters.
- When non-metric units were used in the United Kingdom, a unit of mass called the pound-mass (lbm) was employed, where [latex]1\phantom{\rule{0.25em}{0ex}}\text{lbm}=0\text{.}\text{4539}\phantom{\rule{0.25em}{0ex}}\text{kg}[/latex].
- If there is an uncertainty of [latex]0\text{.}\text{0001}\phantom{\rule{0.25em}{0ex}}\text{kg}[/latex] in the pound-mass unit, what is its percent uncertainty?
- Based on that percent uncertainty, what mass in pound-mass has an uncertainty of 1 kg when converted to kilograms?
- The length and width of a rectangular room are measured to be [latex]3\text{.}\text{955}±0\text{.}\text{005}\phantom{\rule{0.25em}{0ex}}\text{m}[/latex] and [latex]3\text{.}\text{050}±0\text{.}\text{005}\phantom{\rule{0.25em}{0ex}}\text{m}[/latex]. Calculate the area of the room and its uncertainty in square meters.
- A car engine moves a piston with a circular cross section of [latex]7\text{.}\text{500}±0\text{.}\text{002}\phantom{\rule{0.25em}{0ex}}\text{cm}[/latex] diameter a distance of [latex]3\text{.}\text{250}±0\text{.}\text{001}\phantom{\rule{0.25em}{0ex}}\text{cm}[/latex] to compress the gas in the cylinder.
- By what amount is the gas decreased in volume in cubic centimeters?
- Find the uncertainty in this volume.
Glossary
- accuracy
- the degree to which a measured value agrees with correct value for that measurement
- method of adding percents
- the percent uncertainty in a quantity calculated by multiplication or division is the sum of the percent uncertainties in the items used to make the calculation
- percent uncertainty
- the ratio of the uncertainty of a measurement to the measured value, expressed as a percentage
- precision
- the degree to which repeated measurements agree with each other
- significant figures
- express the precision of a measuring tool used to measure a value
- uncertainty
- a quantitative measure of how much your measured values deviate from a standard or expected value
the degree to which a measured value agrees with correct value for that measurement
the degree to which repeated measurements agree with each other
a quantitative measure of how much your measured values deviate from a standard or expected value
the percent uncertainty in a quantity calculated by multiplication or division is the sum of the percent uncertainties in the items used to make the calculation
the ratio of the uncertainty of a measurement to the measured value, expressed as a percentage
express the precision of a measuring tool used to measure a value

