7 A Field Guide to Chemical Reactions
Melanie M. Cooper and Michael W. Klymkowsky
At last we have arrived at the place where many chemistry courses begin: chemical reactions. In this chapter we will examine what a chemical reaction is, which processes are not chemical reactions, how chemical reactions occur, and how they are characterized. We will also look at how molecules come to be reorganized during a chemical reaction. (In Chapter 8, we will look at reaction behaviors in greater detail.)
There are a bewildering array of possible reactions, but the truth is that most chemical reactions fall into a rather limited number of basic types. This is a good thing for the student of chemistry. Recognizing types simplifies our task greatly, and enables us to achieve a greater level of confidence with predicting and explaining the outcomes of chemical reactions. Although each particular reaction differs in its specific molecules and conditions (e.g., temperature, solvent, etc.), some common rules apply. Rather than bombard you with a lot of seemingly unrelated reactions, we will introduce you to the two most common reaction types: acid–base (which as we will see can also be classified as nucleophile/electrophile) and oxidation-reduction. Keep in mind that whatever the reaction type, reactions are systems composed of reactants, products, and the environment in which the reaction occurs. Reactants behave quite differently in the gas phase than in an aqueous or non-aqueous system. High or low temperatures also affect behavior. In the next chapter, we will consider how thermodynamics and kinetics come into play in particular reactions, under specific conditions. This will then lead us to consider equilibrium and non-equilibrium systems.
7.1 Collisions and Chemical Reactions
First we will state the obvious: chemical reactions are linked to change but not all change involves a chemical reaction. When liquid water boils or freezes, it undergoes a change of state (a phase change) but the water molecules are still discrete H2O molecules. In ice, they remain more or less anchored to one another through H-bonding interactions, whereas in liquid and water vapor they are constantly moving with respect to one another and the interactions that occur between the molecules are transient. We can write out this transition in symbolic form as:
H2O (solid) ⇄ H2O (liquid) ⇄ H2O (vapor)
The double arrows mean that the changes are reversible. In this case, reversibility is a function of temperature, which controls whether the interactions between molecules are stable (as in ice), transient (as in liquid water), or basically non-existent (as in water vapor). What you notice immediately is that there are water molecules present in each phase. This helps shed light on the common misconception that bubbles found in boiling water are composed of oxygen and hydrogen. Boiling does not break the bonds in a water molecule, so the bubbles are actually composed of water vapor. That said, within liquid water there is actually a chemical reaction going on: the disassociation of water into –OH and H+ (which we will discuss in more detail shortly). However a naked proton (that is, H+ as discrete entity) does not exist in water. Therefore, this reaction is more accurately written as:
H2O + H2O ⇄ H3O+ + –OH
Here we see the signature of a chemical reaction. The molecules on the two sides of the equation are different; covalent bonds are broken (an O—H bond in one water molecule) and formed (a H—O bond in the other.) All chemical reactions can be recognized in this way. The water dissociation reaction also illustrates how reactions can vary in terms of the extent to which they occur. In liquid water, which has a concentration of about ~55 M, very few molecules undergo this reaction. In fact, in pure water the concentration of H3O+ is only 10-7 M, which is eight orders of magnitude less than the concentration of water molecules. Another interesting feature of this reaction is that it is going in both directions, as indicated by the double arrows ⇄.
Water reacts with itself to form H3O+ + –OH, and at the same time H3O+ + –OH are reacting to generate water molecules. The reaction is at equilibrium, and in this case the position of the equilibrium indicates that the majority of the species in water are actually water molecules.
In contrast, other reactions essentially go to completion (proceed until essentially all the reactants are used up and the reaction is a equilibrium).[1] For example, pure ethanol (CH3CH2OH), is ~17.1 M and it will burn in air (which contains O2). We can write the reaction going to completion as:
CH3CH2OH + 3 O2 ⇄ 2 CO2 + 3 H2O
There is very little ethanol left if this reaction occurs in the presence of sufficient O2.[2] In the real world, the reaction is irreversible because the system is open and both CO2 and H2O escape and are therefore not able to collide with each other – which would be a prerequisite for the reverse reaction to occur. Another interesting feature of the ethanol burning reaction is that pure ethanol can be quite stable in contact with the atmosphere, which typically contains ~20% O2. It takes a spark or a little heat to initiate the reaction. For example, vodka, which is about 50% ethanol, will not burst into flames without a little help! Most reactions need a spark of energy to get them started, but once started, many of them release enough energy to keep them going. As we saw in our discussion of solutions, some reactions release energy (are exothermic) and some require energy (are endothermic). It is important to note that this overall energy change is not related to the spark or energy that is required to get some reactions started. We will return to these ideas in chapter 8.
Another feature of reactions is that some are faster than others. For example, if we add hydrogen chloride gas to water, a reaction occurs almost instantaneously:
HCl(g) + H2O(l) ⇄ H3O+(aq) + Cl–(aq)
Very little time elapses between dissolving the HCl and the reaction occurring. We say the rate of the reaction is fast or instantaneous (in Chapter 8, we will look more closely at reaction rate and what affects it.) In contrast, when iron nails are left out in the weather, they form rust, a complex mixture of iron oxides and hydroxides. This reaction is slow and can take many years, although in hot climates the reaction goes faster. Similarly, when we cook food, the reactions that take place occur at a faster rate than they would at room temperature.
As we have seen previously, bonded atoms are typically more stable than unbonded atoms. For a reaction to occur, some bonds have to break and new ones have to form. What leads to a bond breaking? Why are new bonds formed? What are the factors that affect whether reactions occur, how much energy is released or absorbed, where they come to equilibrium, and how fast they occur? All these questions and more will be addressed in Chapter 8.
But first things first, in order for a reaction to occur, the reacting molecules have to collide. They have to bump into each other to have a chance of reacting at all. An important point to remember is that molecules are not sitting still. They may be moving from one place to another (if they are in liquid or gaseous phase) and/or they are vibrating and rotating. Remember that the temperature of a system of molecules is a function of the average kinetic energy of those molecules. Normally, it is enough to define the kinetic energy of a molecule as 1/2 mv2, but if we are being completely rigorous this equation applies only to monatomic gases. Molecules are more complex because they can flex, bend, rotate around bonds, and vibrate. Many reactions occur in solution where molecules are constantly in contact with each other—bumping and transferring energy, which may appear as either kinetic or vibrational energy. Nevertheless, we can keep things simple for now as long as we remember what simplifications we are assuming. Recall that although temperature is proportional to the average kinetic energy of the molecules, this does not mean that all the molecules in the system are moving with the same velocity. There is typically a broad range of molecular velocities, even if all the molecules are of the same type. There is an even broader range in reaction mixtures, which have more than one type of molecule in them. Since the system has only a single temperature, all types of molecules must have the same average kinetic energy, which means that the more massive molecules are moving more slowly, on average, than the less massive molecules. At the same time, all the molecules are (of course) moving so they inevitably collide with one another and, if the system has a rigid boundary, with the boundary. We have previously described the distribution of velocities found in the system in terms of a distribution of velocity (or speed) and the percent or even absolute number of molecules with that speed, the Boltzmann distribution. At any particular temperature, there are molecules that move much faster (have higher kinetic energy) and other molecules that move much slower (have less kinetic energy) than the average kinetic energy of the population. This means that when any two molecules collide with one another, the energetics of that interaction can vary dramatically. Some collisions involve relatively little energy, whereas others involve a lot!
These collisions may or may not lead to a chemical reaction, so let’s consider what happens during a chemical reaction. To focus our attention, we will consider the specific reaction of hydrogen and oxygen gases to form water:
2H2 + O2 ⇄ 2H2O
This is, in fact, a very complex reaction, so let’s simplify it in a way that may seem cartoonish but which is, nevertheless, accurate. If we have a closed flask of pure oxygen, and we add some hydrogen (H2) to the flask, the two types of gas molecules quickly mix, because – as you will recall – the mixed system is more probable (that is the entropy of the mixed gases is higher than the unmixed.) Some of the molecules collide with each other, but the overwhelming majority of these collisions are unproductive. Neither the hydrogen molecule (H2) nor the oxygen molecule (O2) are altered, although there are changes in their respective kinetic energies. However, when we add kinetic energy (say, from a burning match, which is itself a chemical reaction), the average kinetic energy of the molecules in the heated region increases, thus increasing the energy that can be transferred upon collision, which increases the probability that a particular collision will lead to a bond breaking, which therefore increases the probability of the H2 + O2 reaction. In addition, because the stability of the bonds in H2O is greater than those of H2 and O2, the reaction releases energy to the surroundings. This energy can take the form of kinetic energy (which leads to a further increase in the temperature) and electromagnetic energy (which results in the emission of photons of light.) In this way, the system becomes self-sustaining. It no longer needs the burning match because the energy released as the reaction continues is enough to keep new molecules reacting. The reaction of H2 and O2 is explosive (it rapidly releases thermal energy and light), but only after that initial spark has been supplied.
We can plot out the behavior of the reaction, as a function of time, beginning with the addition of the burning match. It is worth keeping in mind that the reaction converts H2 and O2 into water. Therefore, the concentrations of H2 and O2 in the system decrease as the reaction proceeds while the concentration of H2O increases. As the reaction proceeds, the probability of productive collisions between H2 and O2 molecules decreases simply because there are fewer H2 and O2 molecules present. We can think of it this way: the rate at which the reaction occurs in the forward (to the right) direction is based on the probability of productive collisions between molecules of H2 and O2. This in turn depends upon their relative concentration (this is why hydrogen will not burn in the absence of O2). As the concentrations of the two molecules decrease, the reaction rate slows down. Normally, the water molecules produced by burning disperse and the concentration (molecules per unit volume) of H2O never grows very large. But if the molecules are in a container, then their concentrations increase, and eventually the backward reaction could begin to occur. The reaction will reach equilibrium, at which point the rate of forward and backward reactions would be equal. Because the forward reaction is so favorable, some (but very little) H2 and O2 would remain at equilibrium. The point is to recognize that reactions are dynamic and, depending on the conditions, the exact nature of the equilibrium state will be determined by concentrations, temperatures, and the nature of the reaction.
Questions
Questions to Answer
- In your own words, define the term chemical reaction. How can you tell when a chemical reaction has occurred?
- Give some examples of reactions that you already know about or have learned about in previous courses.
- What do we mean by rate of reaction? How might you determine a reaction rate?
- What conditions must exist in order for something to react?
- How does the concentration of reactants and products influence the rate of a reaction?
- Are chemical reactions possible in the solid phase?
- What factors are required for a reaction to reach a stable (albeit dynamic) equilibrium?
- Why is a burning building unlikely to reach equilibrium?
- Assuming you have encountered them before, define the terms acidic and basic in your own words.
Questions to Ponder
- What reactions are going on around you right now?
- What is required in order for a reaction to go backwards?
7.2 Acid–Base Reactions: A Guide for Beginners
Let us begin with the hydrogen chloride and water reaction from the last chapter, a classic acid–base reaction. To understand how these types of reactions are related, we need to learn how to identify their essential and common components. Our first hurdle is the fact that the terms acid and acidity, and to a lesser extent, bases and basicity, have entered the language of everyday life. Most people have some notion of acids and acidity. Examples of common usage include: acid rain, stomach acid, acid reflux, acid tongue, etc. You might hear someone talk about wine that tastes acidic, by which they probably mean sour, and most people would nod their heads in comprehension. Old wine tastes like vinegar because it contains acetic acid. You have also probably heard of or even learned about measurements of acidity that involve pH, but what is pH exactly? What is an acid, and why would you want to neutralize it? Are acidic things bad? Do we need to avoid them at all costs and under all circumstances? Although the term base is less common, you may already be familiar with materials that are basic in the chemical sense. Bases are often called alkalis, as in alkaline batteries and alkali metals. They are slippery to the touch, bitter tasting.
Not surprisingly, many definitions of acid–base reactions have been developed over the years. Each new definition has been consistent, that is it produces similar conclusions when applied to a particular system, to the ones that have come before, but each new definition has also furthered the evolution of the idea of acids and bases. Later definitions encompass original ideas about acids and bases, but also broaden them and make them more widely applicable, covering a large array of reactions with similar characteristics. We will start with the simplest model of acids and bases—the Arrhenius model.[3] This is the most common introduction to acid–base chemistry; perhaps you have already been taught this model. Although the Arrhenius model is of limited usefulness, we will examine its simple structure as the foundation for more sophisticated and useful models. Our model-by-model consideration should help you appreciate how acid–base chemistry has become increasingly general, and powerful over time. As we progress, keep this simple rule in mind: All acid–base reactions begin and end with polarized molecules. As we go through the various models for acid–base reactions, see if you can identify the polar groups and how they interact with each other.
Arrhenius Acids and Bases
In the Arrhenius model, an acid is defined as a compound that dissociates when dissolved in water to produce a proton (H+) and a negatively-charged ion (an anion). In fact, naked protons (H+) do not roam around in solution. They always associate with at least one, and more likely multiple, water molecules. [4] Generally, chemists use a shorthand for this situation, either referring to the H+ in aqueous solution as a hydronium ion (denoted as H3O+) or even more simply as H+, but do not forget, this is a short-hand. An example of an Arrhenius acid reaction is:
HCl(g) + H2O ⇄ H3O+ (aq) + Cl– (aq)
or, more simply (and truer to the original theory):
HCl(g) ⇄ H+ (aq) + Cl– (aq) or HCl(aq)
But this is really quite a weird way to present the actual situation, because the HCl molecule does not interact with a single water molecule, but rather interacts with water as a solvent. When hydrogen chloride (HCl) gas is dissolved in water, it dissociates into H+(aq) and Cl–(aq) almost completely. For all intents and purposes, there are no HCl molecules in the solution. An aqueous solution of HCl is known as hydrochloric acid, which distinguishes it from the gas, hydrogen chloride. This complete dissociation is a characteristic of strong acids, but not all acids are strong!
An Arrhenius base is defined as a compound that generates hydroxide (–OH) ions when dissolved in water. The most common examples of Arrhenius bases are the Group I (alkali metal) hydroxides, such as sodium hydroxide:
NaOH(s) + H2O ⇄ Na+(aq) + –OH(aq) or NaOH(aq)
Again, this is a reaction system that involves both NaOH and liquid water. The process of forming a solution of sodium hydroxide is just like the one involved in the interaction between sodium chloride (NaCl) and water: the ions (Na+ and –OH) separate and are solvated (surrounded) by the water molecules.
As we will see shortly, some acids (and bases) do not ionize completely; some of the acid molecules remain intact when they dissolve in water. When this occurs we use double-headed arrows ⇌ to indicate that the reaction is reversible, and both reactants and products are present in the same reaction mixture. We will have much more to say about the duration and direction of a reaction in the next chapter. For now, it is enough to understand that acid–base reactions (in fact, all reactions) are reversible at the molecular level. In the case of simple Arrhenius acids and bases, however, we can assume that the reaction proceeds almost exclusively to the right.
An Arrhenius acid–base reaction occurs when a dissolved (aqueous) acid and a dissolved (aqueous) base are mixed together. The product of such a reaction is usually said to be a salt plus water and the reaction is often called a neutralization reaction: the acid neutralizes the base, and vice versa. The equation can be written like this:
HCl(aq) + NaOH(aq) ⇄ H2O(l) + NaCl(aq)
When the reaction is written in this molecular form it is quite difficult to see what is actually happening. If we rewrite the equation to show all of the species involved, and assume that the number of HCl and NaOH molecules are equal, we get:
H+(aq) + Cl–(aq) + Na+(aq) + –OH(aq) ⇄ H2O(l) + Na+(aq) + Cl–(aq)
Na+(aq) and Cl–(aq) appear on both sides of the equation; they are unchanged and do not react (they are often called spectator ions because they do not participate in the reaction). The only actual reaction that occurs is the formation of water:
H+(aq) + –OH(aq) ⇄ H2O(l)
The formation of water (not the formation of a salt) is the signature of an Arrhenius acid–base reaction. A number of common strong acids, including hydrochloric acid (HCl), sulfuric acid (H2SO2), and nitric acid (HNO2), react with a strong base such as NaOH or KOH (which, like strong acids, dissociate completely in water) to produce water..
Such acid–base reactions are always exothermic and we can measure the temperature change and calculate the corresponding enthalpy change (ΔH) for the reaction. Regardless of which strong acid or strong base you choose, the enthalpy change is always the same (about 58 kJ/mol of H2O produced). This is because the only consistent net reaction that takes place in a solution of a strong acid and a strong base is:
H+ (aq) + –OH (aq) ⇄ H2O(l)
One other factor to note is that the overall reaction involves a new bond being formed between the proton (H+) and the oxygen of the hydroxide (–OH.) It makes sense that something with a positive charge would be attracted to (and bond with) a negatively-charged species (although you should recall why the Na+ and Cl– do not combine to form sodium chloride solid in aqueous solution.) Whether or not bonds form depends on the exact nature of the system, and the enthalpy and entropy changes that are associated with the change. We will return to this idea later in chapter 8.
Questions
Questions to Answer
- What would be the reaction if equal amounts of equimolar HNO3 and KOH were mixed?
- How about equal amounts of equimolar H2SO4 and KOH? What would the products be?
- How about equal amounts of equimolar H3PO4 and KOH?
- How many moles of NaOH would be needed to react fully with one mole of H3PO4?
- Draw a molecular level picture of Arrhenius acid base reaction.
Brønsted–Lowry[5] Acids and Bases
The Arrhenius acid–base model is fairly easy to understand but its application is limited to certain kinds of reactions. Rather than continue down this road, chemists found that they needed to expand their model of acids and bases and how they react. The first of these expansions was the Brønsted–Lowry model. In the Brønsted–Lowry model, an acid is characterized as a proton (H+) donor and a base as a proton acceptor. If we revisit the reactions we looked at earlier in the context of the Brønsted–Lowry acid-base model, we see that HCl is the proton donor; it gives away H+ and water is the proton acceptor. In this scheme, HCl is the acid and water is the base:
HCl(g) + H2O(l) ⇄ H3O+ (aq) + Cl–(aq)
acid base conjugate acid conjugate base
The resulting species are called the conjugate acid (so H3O+ is the conjugate acid of H2O) and the conjugate base (Cl– is the conjugate base of HCl). This is because H3O+ can and generally does donate its H+ to another molecule (most often another water molecule) and Cl– can accept an H+.
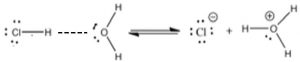
A major (and important difference) between the Brønsted–Lowry and Arrhenius acid–base models is that a Brønsted–Lowry acid must always have an accompanying base to react with—the two are inseparable. A proton donor must have something to donate the protons to (a base)—in this case, water. Remember that bond breaking requires energy, whereas bond formation releases energy. Some energy input is always required for a reaction in which the only thing that happens is the breaking of a bond (for example the Cl–H bond in HCl). Acid–base reactions are typically exothermic; they release energy to the surroundings and the released energy is associated with the interaction between the H+ and the base. In other words, the proton does not drop off the acid and then bond with the base. Instead, the acid–H bond starts to break as the base–H bond starts to form. One way that we can visualize this process is to draw out the Lewis structures of the molecules involved and see how the proton is transferred.
As shown in the figure, we use a dotted line to show the growing attraction between the partial positive charge on the H of the H—Cl molecule and the partial negative charge on the oxygen. This interaction results in the destabilization of the H—Cl bond. Because the Cl is more electronegative than the H, the electrons of the original H—Cl bond remain with the Cl (which becomes Cl–) and the H+ forms a new bond with a water molecule. Essentially, a Brønsted–Lowry acid–base reaction involves the transfer of a proton from an acid to a base, leaving behind the original bonding electrons.
Another example of an acid–base reaction is the reaction of ammonia with water:
NH3(aq) + H2O(l) ⇄ NH4+(aq) + –OH(aq)
base acid conjugate acid + conjugate base

In this case, oxygen is more electronegative than nitrogen. The proton is transferred from the oxygen to the nitrogen. Again, the dotted line in the figure represents the developing bond between the hydrogen and the nitrogen. As the H—O bond breaks, a new H—N bond forms, making the resulting NH4+ molecule positively-charged. The electrons associated with the original H—O bond are retained by the O, making it negatively-charged. So, water is the acid and ammonia is the base! An important difference between this and the preceding HCl–H2O reaction is that H2O is a much weaker acid than is HCl. 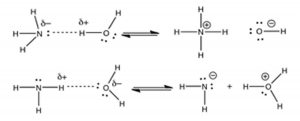 In aqueous solution, not all of the NH3 reacts with H2O to form NH4+. Moreover, the reaction between NH3 and water is reversible, as indicated by the ⇄ symbol. The next chapter will consider the extent to which a reaction proceeds to completion. You may be wondering why the water does not act as a base in the reaction with NH3, like it does with HCl. If you draw out the products resulting from a proton transfer from nitrogen to oxygen, you will see that this process results in a mixture of products where the more electronegative atom (O) now has a positive charge, and the less electronegative atom (N) has a negative charge. It does not make sense that the most electronegative atom would end up with a positive charge, and indeed this process does not happen (to any measurable extent).
In aqueous solution, not all of the NH3 reacts with H2O to form NH4+. Moreover, the reaction between NH3 and water is reversible, as indicated by the ⇄ symbol. The next chapter will consider the extent to which a reaction proceeds to completion. You may be wondering why the water does not act as a base in the reaction with NH3, like it does with HCl. If you draw out the products resulting from a proton transfer from nitrogen to oxygen, you will see that this process results in a mixture of products where the more electronegative atom (O) now has a positive charge, and the less electronegative atom (N) has a negative charge. It does not make sense that the most electronegative atom would end up with a positive charge, and indeed this process does not happen (to any measurable extent).
We will soon return to a discussion of what makes a compound acidic and/or basic. At the moment, we have two acid–base reactions: one in which water is the acid and the other in which water is the base. How can this be? How can one molecule of water be both an acid and a base, apparently at the same time? It is possible because of the water molecule’s unique structure. In fact, water reacts with itself, with one molecule acting as an acid and one as a base:
H2O(l) + H2O(l) ⇄ H3O+ (aq) + –OH(aq)
acid base conjugate acid + conjugate base
As shown in the figure, we can again visualize this process by drawing out the Lewis structures of the water molecules to see how the proton is able to move from one water molecule to another, so that it is never “alone” and always interacting with the lone pairs on the oxygens.
Questions
Questions to Ponder
- Between the Arrhenius model and the Brønsted–Lowry model of acids and base, which is more useful? Why?
Questions to Answer
- Which do you think is more likely to happen? The reaction H2O + H2O → H3O+ + –OH? Or the reverse process H3O+ + –OH → H2O + H2O? Could they both happen at once?
- What do you think the relative amounts of H2O, H3O+ + –OH might be in a pure sample of liquid water? How would you measure the relative amounts?
- Now that you know HCl is an acid and ammonia is a base, can you predict the reaction that occurs between them?
- Is water a necessary component of a Brønsted–Lowry acid–base reaction? How about for an Arrhenius acid–base reaction?
How to Spot an Acid
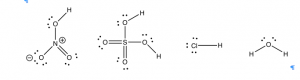
Moving on from water, can we predict whether a compound will be an acid, a base, or neither? We have learned that we can predict many properties of materials by considering their molecular structure. When acids are written in their simplified form (for example HNO3 or H2SO4) it can be very difficult to see any similarities, but if we draw out the Lewis structures some commonalities emerge. Let us take a look at the Lewis structures for several strong acids, such as hydrochloric acid HCl(aq), nitric acid HNO3 (aq), and sulfuric acid H2SO4 (aq).[6] What structural feature do these substances have in common? Well, from their formulae it is clear that they all contain hydrogen, but there are many compounds that contain hydrogen that are not acidic. For example, methane (CH4) and other hydrocarbons are not acidic; they do not donate protons to other molecules.
One common feature of acids is that the proton that gets donated (or picked off) is bonded to a highly electronegative atom. This atom is often either an oxygen or a halogen such as chlorine (Cl), bromine (Br), or iodine (I). Once you know what to look for, it is quite easy to spot the potentially acidic sites in a molecule. For example, in the previous figure, you could circle the “vulnerable” hydrogens. The ability to spot donatable hydrogens is a useful skill that allows you to predict properties of more complex molecules. But why is a hydrogen that is covalently bonded to an electronegative element potentially acidic and donatable?
First, let us consider the O—H bond. Based on our discussion of water molecules, we can predict that it is polarized, with a partial positive charge on the H and a partial negative on the O. In water, the H is (on average) also part of a hydrogen bonding interaction with the oxygen of another water molecule. It turns out that it does not take much energy to break the original O—H bond. Remember that H+ does not just “drop off” the acid, but at the same time forms a bond with the base molecule. In fact, strong acid–base reactions are typically exothermic, meaning that the new bond formed between the proton (H+) and the base is stronger than the bond that was broken to release the H+. The released energy raises the temperature of the surroundings. In an aqueous solution of a strong acid, hydrogen ions are moving rapidly and randomly from one oxygen to another. The energy for all this bond-breaking comes from the thermal motion of water molecules.
We must also consider what happens to the oxygen that gets left behind. When the acidic hydrogen is transferred, it leaves behind the electrons that were in the bond, giving that atom more electrons than it started with. The species left behind must be stable even with those extra electrons (the negative charge). In the example below, chloride ion Cl–(aq) is left behind when the proton gets transferred away. We know chloride is stable and common. It is not surprising that it is one of the products of the reaction.
HCl(g) + H2O(l) ⇄ H3O+ (aq) + Cl–(aq)
acid base conjugate acid + conjugate base
If you recall, electronegativity is a measure of the ability to attract (and retain) electrons.[7] Therefore, it makes sense that a negatively-charged, electronegative atom (like chlorine or oxygen) will be more stable than a negatively-charged, less electronegative atom (like carbon).
Questions
Questions to Answer
- What other atoms besides chlorine or oxygen are electronegative enough to stabilize those extra electrons?
- Draw the reactions of each of the strong acids with water: (HCl(aq)), nitric acid (HNO3 (aq)), sulfuric acid (H2SO4 (aq)), hydrogen bromide (HBr(aq)), and hydrogen iodide (HI(aq)). What are the commonalities? What are the differences?
- Draw the structures of methanol (CH3OH), acetic acid (CH3COOH), and methane (CH4) and write a potential reaction with water. Label the conjugate acid–base pairs.
- Which reactions do you think are likely to occur? Why?
Questions for Later
- What other methods (besides having a strongly electronegative atom) might be available to stabilize the electrons (recall that one model of bonding allows for molecular orbitals that extend over more than two atoms)? We will return to this idea later.
Strong, Weak, Concentrated, and Dilute Acids and Bases
It can be very confusing when words have a different meaning in the scientific context than they do in everyday life. The words we use to describe solutions of acids and bases fall into this category of easily mixed-up definitions. We use the term strong to refer to acids that ionize completely in water, and weak for those acids that are only partially ionized (see Chapter 8 for more information on why). Strong and weak are used to describe an intrinsic property of the acid or base. The terms dilute and concentrated are used to describe the concentration of the acid in water. We could have a dilute solution (say 0.1 M) of the strong acid hydrochloric acid, or a concentrated solution (say 10 M) of the weak acid acetic acid. By contrast, when we refer to strong versus weak liquids in the everyday sense, we are referring to the concentration of the solution. For example, if you say, “This tea is very weak” or “I like my coffee strong” what you are really saying that you like a lot of tea or coffee dissolved in the solution you are drinking. It is important to remember this difference and understand that the scientific context can change the meaning of familiar words.
Questions
Questions to Answer
- Draw out molecular-level pictures of a dilute solution of a strong acid and a weak acid.
- Draw out molecular-level pictures of a concentrated solution of a strong acid and a weak acid.
- What are the similarities and differences between all the representations you have drawn?
- Consider what you have learned about the energy changes associated with the reaction of a strong acid with water. From a safety point of view, which of the following actions makes more sense when diluting a concentrated solution of a strong acid with water? Why?
- A. Add water slowly (dropwise) to the concentrated strong acid or
- B. Add the concentrated strong acid dropwise to water
Factors That Affect Acid Strength
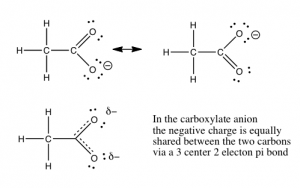
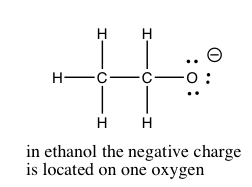 In Chapter 8, we will discuss the quantification of acid and base strength. First let us take a look at the factors that might affect the strength of an acid. As we have already seen, the ability of the conjugate base to hold on to (stabilize) the electron pair is crucial. There are several ways to accomplish this. The simplest is that the acidic H is attached to an electronegative atom such as O, N, or a halogen. There is a wide range of acidities for oxyacids. The differences in acidity are determined by the number of places available for the extra electron density to be stabilized. The figure illustrates a fairly simple example of this in the difference between ethanol (CH3CH2OH) and acetic acid (CH3COOH). Acetic acid is about 10 billion times more acidic than ethanol, because the conjugate base (acetate) is able to stabilize the negative charge on two oxygens instead of just one. This “spreading out” of the charge diminishes the electron-electron repulsions, and stabilizes the structure more than if the negative charge were localized on just one oxygen. If you draw out the Lewis structures of the common strong inorganic oxy-acids (e.g. HNO3 or H2SO4), you will see that it is possible to delocalize the negative charge of the corresponding anion on more than one oxygen.
In Chapter 8, we will discuss the quantification of acid and base strength. First let us take a look at the factors that might affect the strength of an acid. As we have already seen, the ability of the conjugate base to hold on to (stabilize) the electron pair is crucial. There are several ways to accomplish this. The simplest is that the acidic H is attached to an electronegative atom such as O, N, or a halogen. There is a wide range of acidities for oxyacids. The differences in acidity are determined by the number of places available for the extra electron density to be stabilized. The figure illustrates a fairly simple example of this in the difference between ethanol (CH3CH2OH) and acetic acid (CH3COOH). Acetic acid is about 10 billion times more acidic than ethanol, because the conjugate base (acetate) is able to stabilize the negative charge on two oxygens instead of just one. This “spreading out” of the charge diminishes the electron-electron repulsions, and stabilizes the structure more than if the negative charge were localized on just one oxygen. If you draw out the Lewis structures of the common strong inorganic oxy-acids (e.g. HNO3 or H2SO4), you will see that it is possible to delocalize the negative charge of the corresponding anion on more than one oxygen.
How to Spot a Base
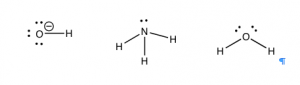 There is an equally simple method for figuring out which compounds are potential bases. Let us take a look at some common bases. The first bases that most people encounter are the metal hydroxides such as NaOH, KOH, and Mg(OH)2. The metal ions are generated when these compounds dissolve in water, but they typically do not play any role in acid–base reactions.[8] The base in these compounds is the hydroxide (–OH). Another common class of bases is molecules that contain nitrogen, like NH3. There many kinds of nitrogenous bases, some of which play a critical role in biological systems. For example, the bases in nucleic acids (DNA and RNA) are basic because they contain nitrogen. Let us not forget that water is also basic and can accept a proton.
There is an equally simple method for figuring out which compounds are potential bases. Let us take a look at some common bases. The first bases that most people encounter are the metal hydroxides such as NaOH, KOH, and Mg(OH)2. The metal ions are generated when these compounds dissolve in water, but they typically do not play any role in acid–base reactions.[8] The base in these compounds is the hydroxide (–OH). Another common class of bases is molecules that contain nitrogen, like NH3. There many kinds of nitrogenous bases, some of which play a critical role in biological systems. For example, the bases in nucleic acids (DNA and RNA) are basic because they contain nitrogen. Let us not forget that water is also basic and can accept a proton.
 So what is the common structural feature in bases? Well, if an acid is the species with a proton to donate, then the base must be able to accept a proton. This means that the base must have somewhere for the proton to attach—it must contain a non-bonded (lone) pair of electrons for the proton to interact and bond with. If we look at our examples so far, we find that all the bases have the necessary non-bonded pair of electrons. Most common bases have either an oxygen or a nitrogen (with lone pairs of electrons) acting as the basic center. Once you learn how to spot the basic center, you can predict the outcome of a vast range of reactions rather than just memorizing them. It is often the case that if you can identify the acidic and basic sites in the starting materials, you can predict the product and ignore the rest of the molecule.
So what is the common structural feature in bases? Well, if an acid is the species with a proton to donate, then the base must be able to accept a proton. This means that the base must have somewhere for the proton to attach—it must contain a non-bonded (lone) pair of electrons for the proton to interact and bond with. If we look at our examples so far, we find that all the bases have the necessary non-bonded pair of electrons. Most common bases have either an oxygen or a nitrogen (with lone pairs of electrons) acting as the basic center. Once you learn how to spot the basic center, you can predict the outcome of a vast range of reactions rather than just memorizing them. It is often the case that if you can identify the acidic and basic sites in the starting materials, you can predict the product and ignore the rest of the molecule.
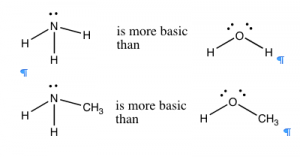
In general, nitrogen is a better proton acceptor than oxygen, because it is more basic. Ammonia (NH3) is more basic than water (H2O), and organic compounds with nitrogen in them are typically more basic than the corresponding compounds containing structurally-analogous oxygens (→). If we compare the trend in basicity for a range of simple compounds across the periodic table, we see that basicity decreases from NH3 > H2O > HF. This effect parallels the increase in electronegativity across the row. The ability to of an electron pair to bond with and accept a proton depends on how tightly that electron pair is held in by the donor atom. In fluorine, the most electronegative atom, the electrons are held so tightly and so close to the atom’s nucleus that they are not available to bond with a proton. Oxygen holds onto its electron pairs a little less tightly, and so is more likely than fluorine to donate a lone pair to a proton. Nitrogen, however, is even less electronegative and therefore has a more available lone pair, making most nitrogen compounds basic.[9]
Questions
Questions to Answer
- Why did we not include CH4 or neon in this analysis?
- Do you think compounds with ammonium (NH4+) are basic? Why or why not?
- Can you draw the structure of a basic compound that has not yet been mentioned in the text?
- Draw out the reactions of CH3NH2 and CH3OH with water. Label the conjugate acid and base pairs. Which reaction is most likely to occur? Why?
- How would you design an experiment to figure out whether a compound is an acid or a base (or both)? What experimental evidence would you accept to determine if you had an acid or a base or both?
7.3 Lewis Acid–Base Reactions
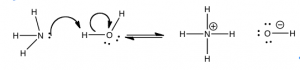 Although chemists use the Brønsted–Lowry model for any reaction in which a proton is transferred from one atom to another, there is an even broader model. The Lewis model incorporates reactions where there is no proton transfer. Instead of seeing the reaction as a proton transfer, we can look at it from the vantage point of the electron pair that eventually becomes part of the new bond. That is: we can consider an acid-base reaction as the donation of an electron pair (from a base) to form a bond between the donor atom and the proton (or the acid).
Although chemists use the Brønsted–Lowry model for any reaction in which a proton is transferred from one atom to another, there is an even broader model. The Lewis model incorporates reactions where there is no proton transfer. Instead of seeing the reaction as a proton transfer, we can look at it from the vantage point of the electron pair that eventually becomes part of the new bond. That is: we can consider an acid-base reaction as the donation of an electron pair (from a base) to form a bond between the donor atom and the proton (or the acid). 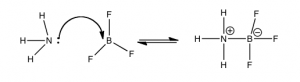 So, instead of saying water transfers a proton to ammonia, the Lewis model would view the process as ammonia donating a lone electron pair to form a new bond with a proton from a water molecule. This process results in the transfer of a hydrogen from the water to the ammonia molecule (a bond formation event, as shown in the figure). The electrons that were originally bonded to the hydrogen do not disappear. Rather, they are left behind on the oxygen, leading to the generation of a hydroxide (–OH) ion. The Lewis acid–base model allows us to consider reactions in which there is no transferred hydrogen, but where there is a lone pair of electrons that can form a new bond.
So, instead of saying water transfers a proton to ammonia, the Lewis model would view the process as ammonia donating a lone electron pair to form a new bond with a proton from a water molecule. This process results in the transfer of a hydrogen from the water to the ammonia molecule (a bond formation event, as shown in the figure). The electrons that were originally bonded to the hydrogen do not disappear. Rather, they are left behind on the oxygen, leading to the generation of a hydroxide (–OH) ion. The Lewis acid–base model allows us to consider reactions in which there is no transferred hydrogen, but where there is a lone pair of electrons that can form a new bond.
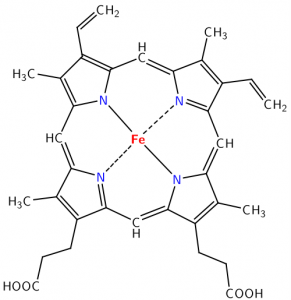
This figure shows an example of the Lewis acid–base model in the reaction between boron trifluoride (BF3) and ammonia (NH3). In this case, the base is the electron pair donor and the acid is the electron pair acceptor. The lone electron pair from NH3 is donated to boron, which has an empty bonding orbital that accepts the pair of electrons, forming a bond between the N and the B. Even though we use the term “donate”, the electron pair does not leave the NH3 molecule; it changes from a non-bonding pair to a bonding pair of electrons. BF3 is a Lewis acid, but note that it has no H to donate. It represents a new class of acids: Lewis acids. These include substances such as BF3 or AlCl3, compounds of periodic table Group III atoms, which have only six electrons in their bonding orbitals. This electron deficiency leaves empty, energetically-accessible orbitals open to accept an electron pair from the Lewis base, the electron pair donor. Other examples of Lewis acids are metal ions, like Fe2+, Fe3+, Mg2+, and Zn2+. All of these elements play a critical role in biological systems via their behavior as Lewis acids. An important example is the heme group of hemoglobin. In the center of this group is a positively-charged iron (Fe) atom. Such positively-charged ions (cations) have empty orbitals that can interact with the lone pair electrons from Lewis bases and form Lewis acid–base complexes. In the case of hemoglobin, the Lewis bases (O2, CO2, and CO) interact with Fe to move oxygen into the body from the lungs and move CO2 from the body to the lungs. It takes a little practice to gain confidence in recognizing Lewis acid–base reactions, but this skill can help us understand many biological and chemical systems.
If we look back over the acid–base theories about acids, we see that the theories become increasingly complex as each subsequent theory subsumes the previous one and extends the range of reactions that can be explained. Neither the Arrhenius nor Brønsted–Lowry theories explain why iron in the heme complexes and oxygen to form the oxygen transport system in our bodies. The Lewis acid–base model, on the other hand, can help explain this as well as the simple reaction between HCl and NaOH (where –OH is the Lewis base and H+ is the Lewis acid).
Questions
Questions to Answer
- For the reaction: HCl(g) + H2O(l) –> H3O+(aq) + Cl–(aq), write out (in words and molecular level pictures) what is going on during the reaction in terms of:
- Arrhenius acid–base theory
- Bronsted–Lowry acid–base theory
- Lewis acid–base theory
- Now do the same activity for the reaction of NH3 and HCl.
- Now do the same activity for the reaction of R2NH and AlCl3.
- Why do you think we use different models of acid–base reactions?
- Can you describe what would dictate the use of a particular model?
7.4 Nucleophiles and Electrophiles
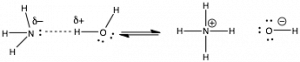
The Lewis acid–base model is more inclusive than the Brønsted–Lowry model, but we often use the Brønsted–Lowry model because it is easier to follow the proton transfer from one molecule (the acid) to another (the base). In aqueous solutions, the Brønsted–Lowry theory also allows us to use the concept of pH to quantify acidity (as we will see shortly). Both the Lewis and Brønsted–Lowry models capture the overarching principle that most chemical reactions are initiated by an electrostatic interaction between a positively-charged portion of a molecule to a negatively-charged portion of the same, or another, molecule.[10] As we will see in the next chapter, molecules must collide with one another in order for reactions to occur between them—they do not react at a distance. When the reacting particles collide, there has to be some continuous pathway through which bonds rearrange and produce products. The first step in this pathway often involves Coulombic (electrostatic) interactions between specific regions of the molecules involved. Of course, whether or not such Coulombic interactions are stable depends upon the kinetic energies of the colliding molecules and exactly how they collide with one another. Catalysts often speed reactions by controlling how molecules collide with or interact with one another. This figure (→) shows the reaction of H2O and NH3, in which the positive end of one molecule interacts with the negative end of the other. If we consider this as a Lewis acid–base reaction, the same principle holds true. It turns out that we can profitably consider a wide range of reactions using the principle of Coulombic attraction. For example, ammonia (and other nitrogen compounds) can react with carbon-containing molecules if the appropriate conditions are met.
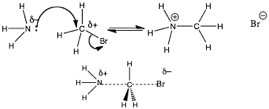
In the figure (→) the nitrogen is behaving as a Lewis base, donating its lone pair of electrons to the carbon. However, it is a little more difficult to see the analogy with a Lewis acid at the carbon site. What we can see is that there is an electronegative, polarizing group (in this case a bromine atom) bonded to the carbon. The presence of a bromine atom polarizes the C—Br bond, giving the carbon a slight positive charge. This makes the carbon susceptible to attack by the lone pair of the nitrogen. Since carbon does not have an empty orbital to accept the lone pair into, and carbon can never form more than four bonds, something has to give. What gives is the C—Br bond, which breaks, and the bromine carries away the electrons from the bond with it, producing a bromide ion, Br–.
This type of reaction, while is essentially a Lewis acid-base reactions, is usually described using yet another set of terms, probably because these reactions usually belong in the realm of organic chemistry, which was once considered a distinct chemical discipline. For organic chemists, the species with the lone pair (in this case the NH3) is called the nucleophile (literally, “nucleus-loving”) and is attracted to a positive center of charge. The species that accepts the lone pair of electrons, in this case the CH3Br molecule, is called the electrophile (literally, “electron-loving”). The species that is released from its bond with the carbon is called the leaving group. Leaving groups must be relatively electronegative (as in the case of Br) or stable when associated with an extra pair of electrons. So, good leaving groups are weak bases. Conjugate bases of strong acids are excellent leaving groups because they are stable.
If we analyze the reaction in the figure further, we see the nitrogen nucleophile approaching the carbon electrophile: as the bond forms between the C and N, the bond breaks between the C and the Br. The bond-breaking and bond-making occur simultaneously. Given what we know about water and aqueous solutions, we might even be so brave as to predict that the product (+NH3CH3 Br–) will rapidly lose a proton in aqueous solution to produce CH3—NH2 and H3O+. This kind of reaction is often referred to as a methylation (a –CH3 group is a methyl group). The product is an N-methylated derivative of ammonia.
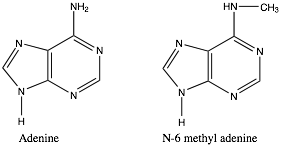 As we have already seen, nitrogen compounds are common in biological systems. We now see how these compounds can also act as nucleophiles, and how methylation of nitrogen is a fairly common occurrence with a range of effects. For example, methylation and demethylation of the nitrogenous bases in DNA adenine and cytosine is used to influence gene expression and mark newly synthesized DNA strands from older, preexisting DNA strands. At the same time, various methylated sequences (such as CpG) are much less stable than the unmethylated form, and so more likely to to mutate.[11] Methylation reactions are quite common in other biological reactions as well. For example, epinephrine (also known as adrenaline, the fight-or-flight hormone) is synthesized in the body by methylation of the related molecule norepinephrine.
As we have already seen, nitrogen compounds are common in biological systems. We now see how these compounds can also act as nucleophiles, and how methylation of nitrogen is a fairly common occurrence with a range of effects. For example, methylation and demethylation of the nitrogenous bases in DNA adenine and cytosine is used to influence gene expression and mark newly synthesized DNA strands from older, preexisting DNA strands. At the same time, various methylated sequences (such as CpG) are much less stable than the unmethylated form, and so more likely to to mutate.[11] Methylation reactions are quite common in other biological reactions as well. For example, epinephrine (also known as adrenaline, the fight-or-flight hormone) is synthesized in the body by methylation of the related molecule norepinephrine.
Considering Acid–Base Reactions: pH
It is almost certain that you have heard the term pH, it is another of those scientific terms that have made it into everyday life, yet its scientific meaning is not entirely obvious. For example: why does an increase in pH correspond to a decrease in “acidity” and why does pH change with temperature?[12] How do we make sense of pH and use that information to better understand chemical systems?

The key idea underlying pH is that water undergoes an acid–base reaction with itself. Recall that this reaction involves the transfer of a proton from one water molecule to another. The proton is never free or “alone”; it is always bonded to an oxygen within another water molecule. Another important point about pH is that the reaction is readily reversible. Under normal conditions (room temperature), the reaction proceeds in both directions. If we look at the reaction, it makes intuitive sense that the reactants on the right (H3O+ and –OH) can react together to give two H2O molecules simply because of the interaction of the positive and negative charges, and we have already seen that the forward reaction does occur. This is one of the first examples we have seen of a reaction that goes both forward and backward in the same system. As we will see, all reactions are reversible at the nanoscale (we will consider the implications of this fact in detail in the next chapter). In any sample of pure water, there are three different molecular species: water molecules (H2O), hydronium ions (H3O+), and hydroxide ions (–OH), as shown in the figure (→). These three species are constantly interacting with each other through the formation of relatively weak H-bonding interactions, which are constantly forming and breaking. Remember, in liquid water, the water molecules are constantly in motion and colliding with one another. Some of these collisions have enough energy to break the covalent H—O bond in water or in the hydronium ion. The result is the transfer of H+ and the formation of a new bond with either another water molecule (to form hydronium ion) or with a hydroxide ion (to form a water molecule). To get a feeling for how dynamic this process is, it is estimated that the average lifetime of an individual hydronium ion is on the order of 1 to 2 picoseconds (1 x 10–12 ps), an unimaginably short period of time. In pure water, at 25 °C, the average concentration of hydronium ions is 1 x 10–7 mol/L. We use square brackets to indicate concentration, so we write this as:
[H3O+] = 1 x 10–7 M
Note that this is a very, very, very small fraction of the total water molecules, given that the concentration of water molecules [H2O] in pure water is ~55.4 M.
In pure water, every time a hydronium ion is produced, a hydroxide ion must also be formed. Therefore, in pure water at 25 °C, the following equation must be true:
[H3O+] = [–OH] = 1 x 10–7 M
It must also be true that the product of the hydronium and hydroxide ion concentrations, [H3O+][–OH], is a constant at a particular temperature. This constant is a property of water. At 25 ºC, this constant is 1 x 10–14 and given the symbol Kw,25ºC. So why do we care? Because when we add an acid or a base to a solution of water at 25 ºC, the product of [H3O+][–OH] remains the same: 1 x 10–14. We can use this fact to better understand the behavior of acids, bases, and aqueous solutions.
For many people, dealing with negative exponents does not come naturally. Their implications and manipulations can be difficult. Believe it or not, the pH scale[13] was designed to make dealing with exponents easier, but it does require that you understand how to work with logarithms (perhaps an equally difficult task). pH is defined as: pH = – log [H3O+].[14]
In pure water (at 25 ºC), where the [H3O+] = 1 x 10–7 M, pH = 7 (pH has no units). A solution with a higher concentration of hydronium ions than pure water is acidic, and a solution with a higher concentration of hydroxyl ions is basic. This leads to the counter-intuitive fact that as acidity [H3O+] goes up, pH goes down. See for yourself: calculate the pH of a solution with a [H3O+] of 1 x 10–2 M (pH = 2), and of 1 x 10–9 M (pH = 9). Moreover, because it is logarithmic, a one unit change in pH corresponds to a change in [H3O+] of a factor of 10.
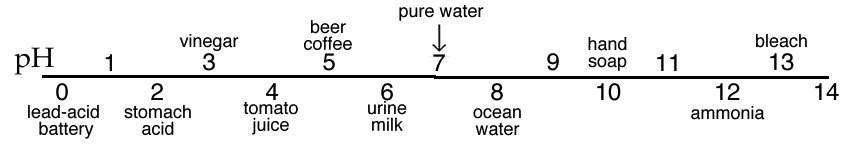
The pH scale is commonly thought of as spanning units 1–14, but in fact many of the strongest acid solutions have pH < 1. Representations of the pH scale often use colors to indicate the change in pH. This convention is used because there are many compounds that change color depending on the [H3O+] of the solution in which they are dissolved. For example, litmus[15] is red when dissolved in an acidic (pH < 7) solution, and blue when dissolved in a basic (pH > 7) solution. Perhaps you have noticed that when you add lemon juice (acidic) to tea, the color changes. Do not get confused: solutions of acids and bases do not intrinsically differ in terms of color. The color change depends on the nature of molecules dissolved in the solution. Think about how changes in pH might affect molecular structure and, by extension, the interactions between molecules and light (a topic that is more extensively treated in the spectroscopy supplement).
It is important to note that at 37 ºC the value of Kw is different: [H3O+][–OH] = 2.5 x 10–14 and therefore the pH = 6.8. Weirdly, this does not mean that the solution is acidic, since [H3O+] = [–OH]. The effect is small, but it is significant; it means that a pH of 7 does not always mean that a solution is neutral (it depends on the temperature). This is particularly important when the concept of pH is applied to physiological systems, since the body is usually not at room temperature.
Now let us consider what happens when we add a Brønsted–Lowry acid to water.

For example, if we prepare a solution of 0.10 M HCl (where we dissolve 0.10 mol HCl(g) in enough water to make 1 liter of solution), the reaction that results (see figure) contains more hydronium ion (H3O+). Now if we measure[16] the pH of the solution of 0.10 M HCl, we find that it is 1.0 pH units. If we convert back to concentration units from pH (if pH = – log [H3O+], then [H3O+] = 10–pH), we find that the concentration of H3O+ in 0.10 M HCl is 0.10 M. This makes sense, in light of our previous discussion about how HCl completely dissociates into Cl– and H+ (associated with water molecules).
| [HCl] M | [H2O] M | [H3O+] M | [OH] M | [Cl–] M | |
| Before reaction | 0.10 | ~55.5 | 1.0 x 10–7 | 1.0 x 10–7 | 0 |
| After Reaction | ~0 | ~55.4 | ~1.0 x 10–1 | 1.0 x 10–13 | 1.0 x 10–1 |
This table gives the concentrations of all the species present both before and after the reaction. There are several things to notice about this table. Because the measured pH = 1 and we added 0.1 M (or 10-1 M) HCl, it is reasonable to assume that all the HCl dissociated and that the vast majority of the H3O+ came from the HCl. We can ignore the H3O+ present initially in the water. Why? Because it was six orders of magnitude (0.0000001)(10-7) smaller than the H+ derived from the HCl (10-1). It is rare to see pH measurements with more than three significant figures, so the H3O+ originally present in the water does not have a significant effect on the measured pH value. Although we are not generally concerned about the amount of hydroxide, it is worth noting that [H3O+][–OH] remains a constant (Kw),and therefore when [H3O+] increases the [–OH] decreases.
Although a number of substances dissolve in water, not all ionize, and not all substances that ionize alter the pH. For example, NaCl ionizes completely when dissolved in water, yet the pH of this solution is still 7. The Na+ and Cl– ions do not affect the pH at all. However, if we make a 1 M solution of ammonium chloride (NH4Cl), we find that its pH is around 5. Although it might not be completely obvious why the pH of this solution is 5 and the pH of a 1M NaCl solution is 7, once you know that it is (and given what you know about pH), you can determine the concentrations of H3O++, NH4+, NH3, –OH and Cl– present (see Chapter 8). The question is: Why are NH4Cl and HCl so different? (We consider this point in Chapter 9.)
Making Sense of Vinegar and Other Acids
Now let us consider another common acid: acetic acid. If wine is left open to the air, it will often begin to taste sour because the ethanol in wine reacts with oxygen in the air and forms acetic acid. Acetic acid belongs to a family of organic compounds known as carboxylic acids. It has one acidic proton attached to the oxygen.

If we measure the pH of a 0.10-M solution of acetic acid, we find that it is about 2.8. The obvious question is why the pH of a 0.10-M solution of acetic acid is different from the pH of a 0.10-M solution of hydrochloric acid? The explanation lies in the fact that acetic acid (CH3COOH) does not dissociate completely into CH3CO2– and H3O+ when it is dissolved in water. A pH of 2.8 indicates that the [H3O+] = 10–2.8. This number can be converted into 1.6 x 10–3 M. About 1.6% of the added acetic acid is ionized (a form known as acetate ion, CH3COO–). The rest is in the protonated form (acetic acid, CH3COOH). The specific molecules that are ionized changes all the time; protons are constantly transferring from one oxygen to another. You can think of this process in another way: it is the system that has a pH, not individual molecules. If we look at a single molecule of acetic acid in the solution, we find that it is ionized 1.6% of the time. This may seem a weird way to think about the system, but remember, many biological systems (such as bacteria) are quite small, with a volume of only a few cubic microns or micrometers (a cubic micron is a cube 10–6 m on a side) and may contain a rather small number of any one type of molecule. Thus, rather than thinking about the bulk behavior of these molecules, which are relatively few, it can be more useful to think of the behavior of individual molecules averaged over time. Again, in an aqueous solution of acetic acid molecules, most of the molecules (~98.4%) are in the un-ionized form, so any particularly molecule is un-ionized ~98.4% percent of the time.
We can measure the pH of the solutions of many acids of known concentrations, and from these measurements make estimates of the strength of the acid. Strong acids, such as nitric, sulfuric, and hydrochloric are all totally ionized in solution. Weaker acids, such as organic acids, ionize to a much lesser extent. However, given the low naturally occurring concentrations of hydronium and hydroxide ions in pure water, even weak acids can significantly alter the pH of an aqueous solution. The same behavior applies to weak bases.
Conversely, if weak acids or bases are dissolved in solutions of different pH, the amount of ionization of the group may be significantly changed. For example, as we will see in chapters 8 and 9, if we added a weak acid to a solution that was basic (for example at pH 9), we would find that much more of the acid will ionize. Many biological molecules contain parts (called functional groups) that behave as weak acids or weak bases. Therefore, the pH of the solution in which these molecules find themselves influences the extent to which these functional groups are ionized. Whether a part of a large molecule is ionized or not can dramatically influence a biomolecule’s behavior, structure, and interactions with other molecules. Thus, changes in pH can have dramatic effects on a biological system. For example, if the pH of your blood changes by ± 0.3 pH units, you are likely to die. Biological systems spend much of the energy they use maintaining a constant pH (typically around 7.35-7.45).[17] In addition, the pH within your cells is tightly regulated and can influence cellular behavior.[18]
Questions
Questions to Answer
- How would you calculate the molarity of pure water?
- What percentage of water molecules are ionized at 25 °C?
- If the pH of a solution (at 25 ºC) is 2.0, what is the [H3O+]? What is the [–OH]?
- If the pH of a solution (at 37 ºC) is 2.0, what is the [H3O+]? What is the [–OH]?
- What would be the pH of a 0.01-M solution of HCl at 25 ºC?
- If the pH of a 0.1-M solution of NH4Cl is 5.1, what is the [H3O+]?
- Draw out a molecular level picture of what you imagine solutions of NaCl and NH4Cl look like.
- Why does acetic acid only have one acidic proton (after all, it does have a total of four protons)?
- Why is acetic acid more acidic than ethanol? What is it about the structure of acetic acid that makes it more acidic?
Questions for Later
- Why do you think we keep specifying the temperature in our discussions of reactions?
Questions to Ponder
- Carboxylic acid groups, –COOH, are common in large biomolecules. What would be the effect of raising or lowering the pH on carboxylate side chains?
- What effect do you think that might have on the properties of the biomolecule (solubility, interactions with other molecules, etc.)?
- Amino groups are also common. What would be the effect of raising or lowering the pH on an amino group?
7.5 Oxidation–Reduction Reactions
In contrast to acid–base reactions, oxidation–reduction (or redox) reactions obey a different pattern. In the simplest kinds of redox reactions, polar products are generated from non-polar reactants. You may have run into such reactions already (even if you did not know what they were called!) When iron is left in contact with oxygen (in air) and water, it rusts. The iron is transformed from a hard, non-polar metallic substance, Fe (solid), into a powdery substance, Fe2O3.nH2O(s). Rusting is mechanistically similar to the reactions that occur when copper turns green, when silver tarnishes and turns black, or (in perhaps the favorite reaction of chemists everywhere[19]) when sodium metal explodes in water.[20]
All of these reactions start with a metal in its elemental form. Pure metals have no charge or permanent unequal distribution of charge (which makes them different from salts like NaCl). In fact we can use the synthesis of sodium chloride (NaCl) from its elements sodium (Na) and chlorine (Cl2) to analyze what happens during a redox reaction. The reaction can be written as:
2Na(s) + Cl2(g) ⇄ 2NaCl(s)
We have already looked at the structure of ionic compounds in Chapter 4 and know that the best way to think about them is to consider NaCl as a three-dimensional lattice of alternating positive (Na+) and negative (Cl–) ions. That is as the reaction proceeds the metal atoms becomes cations, and the chlorine molecules become anions. We could write this as two separate reactions: The Na loses an electron – a process that we define as oxidation.
Na ⇄ Na+ +e– (an oxidation reaction)
The electrons must go somewhere (they cannot just disappear) and since chlorine is an electronegative element, it makes sense that the electrons should be attracted to the chlorine. We define the gain of electrons as a reduction.
Cl + e– ⇄ Cl – (a reduction reaction).
It turns out that all reactions in which elements react with each other to form compounds are redox reactions. For example, the reaction of molecular hydrogen and molecular oxygen is also a redox reaction:
2H2(g) + O2(g) ⇄ 2H2O(l)
The problem here is that there is no obvious transfer of electrons. Neither is there an obvious reason why these two elements should react in the first place, as neither of them has any charge polarity that might lead to an initial interaction. That being said, there is no doubt that H2 and O2 react. In fact, like sodium and water, they react explosively.[21] When we look a little more closely at the reaction, we can see that there is a shift in electron density on individual atoms as they move from being reactants to being products. The reactants contain only pure covalent (H—H and O—O) bonds, but in the product (H2O) the bonds are polarized: Hδ+ and Oδ– (recall that oxygen is a highly electronegative atom because of its highly effective nuclear charge.) There is a shift in overall electron density towards the oxygen. This is a bit subtler than the NaCl case. The oxygen has gained some extra electron density, and so been reduced, but only partially – it does not gain the whole negative charge. The hydrogen has also been oxidized by losing some electron density. We are really talking about where the electron spends most of its time. In order to keep this straight, chemists have developed a system of oxidation numbers to keep track of the losses and gains in electron density.
Oxidation States and Numbers
Now, we may seem to be deploying more arcane terms designed to confuse the non-chemist, but in fact, oxidation numbers (or oxidation states) can be relatively easy to grasp as long as you remember a few basic principles:[22]
- For an ion, the charge is the oxidation number. The oxidation number of Na+ is +1, the oxidation number of the oxide ion (O2–) is –2.
- For elements that are covalently bonded to a different element, we imagine that all the electrons in the bond are moved to the most electronegative atom to make it charged. As an example, the oxygen in water is the more electronegative atom. Therefore, we imagine that the bonding electrons are on oxygen and that the hydrogen atoms have no electrons (rather, they have a +1 charge). The oxidation number of H (in water) is +1, whereas in oxygen it is -2, because of the -2 charge of the two imagined extra electrons that came from the bond.
- Elements always have an oxidation number of zero (because all of the atoms in a pure element are the same, so none of the bonds are polar).
Remember this is just a way to keep track of the electrons. Oxidation numbers are not real; they are simply a helpful device. It is also important to remember that the oxidation number (or state) of an atom is dependent upon its molecular context. The trick to spotting a redox reaction is to see if the oxidation number of an atom changes from reactants to products. In the reaction:
2H2(g) + O2(g) ⇄ 2H2O(l)
H changes from zero in the reactants (H2) to +1 in the products (H2O), and the oxygen goes from zero (O2) to –2 (H2O). When oxidation numbers change during a reaction, the reaction is a redox reaction.
Now lets look at the reaction sodium and water, which is a bit more complicated to see if we can spot what is oxidized and what is reduced.
2Na(s) + 2H2O(l) ⇄ 2Na+(aq) + 2–OH(aq) + H2(g)
It is relatively easy to see that the sodium gets oxidized, because it loses an electron, going from Na to Na+. But which species gets reduced? Is it the oxygen or the hydrogen? Or could it be both? If we check for changes in oxidation state, the oxygen in water starts at –2 and in hydroxide (–OH) it is still –2 (it has not been reduced or oxidized). If we check the hydrogens, we see two distinct fates. One of the hydrogen atoms stays bonded to the oxygen atom (in hydroxide); it starts at +1 and stays there. However, the other type ends up bonded to another hydrogen atom; it starts at +1 and ends at zero. It is these latter two hydrogen atoms that have been reduced!
Historically, the term oxidation has denoted a reaction with oxygen. For example, in simple combustion reactions:
CH4(g)+ O2(g) ⇄ CO2(g) + H2O(g)
Oxidation reactions like this provide major sources of energy, in the burning of fuel (natural gas, gasoline, coal, etc.) and also in biological systems. In the latter, carbons containing molecules such as sugars and lipids react with molecular oxygen to form compounds with very stable bonds (CO2 and H2O), releasing energy that can be used to break bonds and rearrange molecules. In a similar vein the original meaning of reduction was reaction with hydrogen, for example acetic acid can be reduced to ethanol by reacting with hydrogen:
CH3CO2H + H2(g) ⇄ CH3CH2OH
What is important to note is that, there cannot be an oxidation without a reduction – and vice-versa. Just like there can be no acid without a base.
Questions
Questions to Answer
- For the reaction CH4(g)+ O2(g) ⇄ CO2(g) + H2O(g), which atoms are oxidized and which are
- reduced?
- For the reaction CH3CO2H + H2(g) ⇄ CH3CH2OH which atoms are oxidized and which are
reduced? - Write an explanation to a friend who has no chemistry background to explain the difference
between these two reactions that give the same product:
2H2(g) + O2(g) ⇄ 2H2O(l) and H+(aq) + –OH(aq) ⇄ H2O(l)
Questions for Later
- Is it possible to separate out the oxidation reaction (where electrons are lost) and the reduction reaction (where electrons are gained)? What would happen?
- What if you separate the two reactions but join them by an electrical connection? What do you think would happen?
7.6 Energy Changes and Chemical Reactions
All chemical reactions are accompanied by energy changes. Under most circumstances, particularly when the pressure and volume are kept constant, these changes can be ascribed to changes in enthalpy ΔH. For example, combustion reactions (redox reactions involving oxygen) are a major source of energy for most organisms. In warm-blooded organisms, the energy released through such reactions is used to maintain a set body temperature. Within organisms, combustion reactions occur in highly-controlled stages (which is why you do not burst into flames), through the process known as respiration (different from breathing, although breathing is necessary to bring molecular oxygen to your cells).
Not all biological forms of respiration use molecular oxygen.[23] There are other molecules that serve to accept electrons; this process is known as anaerobic (air-free) respiration. All known organisms use the molecule adenosine triphosphate (ATP) as a convenient place to store energy. ATP is synthesized from adenosine diphosphate (ADP) and inorganic phosphate. As two separate species, ADP and inorganic phosphate are more stable than ATP and the energy captured from the environment use to drive the synthesis of ATP can be released again via the formation of ADP and inorganic phosphate:
ADP + Pi + energy ⇄ ATP + H2O
If we looked closely at the molecular level mechanism of ATP synthesis, we would see that it is another example of an electrophile–nucleophile interaction. But regardless of the type of reactions, we can ask the same question: Where (ultimately) does the energy released in an exothermic reaction come from? When an exothermic reaction occurs and energy is transferred from the system to the surroundings, the result is a temperature increase in the surroundings and a negative enthalpy change –ΔH.) What is the source of that energy? Of course, you already know the answer—it has to be the energy released when a bond is formed!
The defining trait of a chemical reaction is a change in the chemical identity of the reactants: new types of molecules are produced. In order for this to occur, at least some of the bonds in the starting material must be broken and new bonds must be formed in the products, otherwise no reaction occurs. So to analyze energy changes in chemical reactions, we look at which bonds are broken and which are formed, and then compare their energies. As we will discuss later, the process is not quite so simple, given that the pathway for the reaction may include higher energy intermediates. As we will see it is the pathway of a reaction that determines its rate (how fast it occurs), whereas the difference between products and reactions determines the extent to which the reaction will occur. The following analysis will lead to some reasonable approximations for estimating energy changes during a reaction.
As we have already seen, bond formation releases energy and bond breaking requires energy. Tables of bond dissociation energies are found in most chemistry books and can be easily retrieved from the Internet.[24] One caveat: these measurements are typically taken in the gas phase and refer to a process where the bond is broken homolytically (each atom in the original bond ends up with one electron and the species formed are known as radicals).[25] The bond dissociation energy for hydrogen is the energy required to drive the process:
H–H(g) ⇄ 2H•
where the dot represents an unpaired electron. The enthalpy change for this process is
ΔH = + 436 kJ/mol. Note that tables of bond energies record the energy required to break the bond. As we noted earlier, enthalpy is a state function – its value does not depend on the path taken for the change to occur, so we also know what the enthalpy change is for the reverse process. That is, when a hydrogen molecule forms from two hydrogen atoms the process is exothermic:
2H • ⇄ H–H(g) ΔH = – 436 kJ/mol.
We have tables of bond energy values for most common bond types, so one way to figure out energy changes (or at least the enthalpy changes) for a particular reaction is to analyze the reaction in terms of which bonds are broken and which bonds are formed. The broken bonds contribute a positive term to the total reaction energy change whereas bond formation contributes a negative term. For example, let us take a closer look at the combustion of methane:[26]
CH4(g)+ 2O2(g) ⇄ CO2(g) + 2H2O(g)
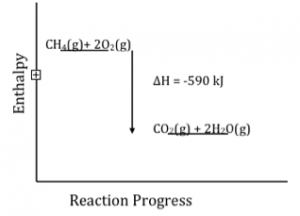 In the course of this reaction, four C—H bonds [4 x C—H (436kJ/mol)] and two O=O bonds (498 kJ/mol) are broken. The new bonds formed are 2 x C=O (803 kJ/mol) and 4 x O—H (460 kJ/mol). If you do the math, you will find that the sum of the bond energies broken is 2740 kJ, whereas the sum of the bond energies formed is –3330 kJ. In other words, the bonds in the products are 706 kJ more stable than the bonds in the reactants. This is easier to see if we plot the progress of enthalpy versus reaction; it becomes more obvious that the products are lower in energy (more stable).
In the course of this reaction, four C—H bonds [4 x C—H (436kJ/mol)] and two O=O bonds (498 kJ/mol) are broken. The new bonds formed are 2 x C=O (803 kJ/mol) and 4 x O—H (460 kJ/mol). If you do the math, you will find that the sum of the bond energies broken is 2740 kJ, whereas the sum of the bond energies formed is –3330 kJ. In other words, the bonds in the products are 706 kJ more stable than the bonds in the reactants. This is easier to see if we plot the progress of enthalpy versus reaction; it becomes more obvious that the products are lower in energy (more stable).
There are several important aspects to note about this analysis:
- This is only an estimation of the enthalpy change, because (as noted above) bond energies are averages and are measured in the gas phase. In the real world, most reactions do not occur in the gas phase. In solutions, there are all kinds of other interactions (intermolecular forces) that can affect the enthalpy change, but for an initial approximation this method often gives surprisingly good results.
- Remember, every reaction must be considered as a part of the system. Both the reactants and products have to be included in any analysis, as well as the direction of energy transfer between the reaction system and the surroundings.
- An exothermic reaction occurs when the bonds formed are stronger than the bonds that are broken. If we look closely at this calculation, we can see that combustion reactions are so exothermic because they produce carbon dioxide. The bond energy of the carbon—oxygen double bond is very high (although not two times the C—O single bond—can you think why?) The production of CO2 is very favorable from an energy standpoint: it sits in a deep energy well because it has such strong bonds. This point has important ramifications for the world we live in. Carbon dioxide is quite stable; although it can be made to react, such reactions require the input of energy. Large numbers of us expel CO2 into the atmosphere from burning fossil fuels and breathing, at a higher rate than is currently being removed through various types of sequestration processes, including chemical reactions and photosynthesis. You have certainly heard of the greenhouse effect, caused by the build-up of CO2. CO2 is difficult to get rid of because strong bonds give it stability. (Given the notoriety of CO2 in terms of climate change, we will come back to this topic later.)
Questions
Questions to Answer
- Many biology texts refer to energy being released when high-energy bonds in ATP are broken. In light of what you know, is this a reasonable statement? What do these texts really mean?
- Why do you think the enthalpy change for most Brønsted–Lowry acid–base reactions is independent of the nature of the acid or base? (Hint: What is the reaction that is actually occurring?)
- Using tables of bond dissociation energies, calculate the energy change for the reaction of CH2=CH2 + HCl ⇄ CH3CH2Cl. What steps do you have to take to complete this calculation? Make a list.
- If you look up the enthalpy change for this reaction (ΔH°) you will find it is not exactly what you calculated. Why do you think that is? (Hint: This reaction typically takes place in a solvent. What role might the solvent play in the reaction?)
- That said, one might argue that 10-7 M is complete ↵
- Although slight traces of ethanol are still detectable; forensic scientists can detect the presence of substances such as hydrocarbons at the scene of a fire, even though the amounts are extremely small. ↵
- Arrhenius proposed these ideas in 1888 and won a Nobel Prize for his discovery of ionization reactions in solution in 1903. ↵
- http://www.nature.com/nature/journal/v397/n6720/abs/397601a0.html ↵
- This theory was postulated simultaneously by both Brønsted and Lowry in 1923. ↵
- In strong acids, the proton is completely donated to water in aqueous solution (i.e., there is no detectable amount of un-ionized acid in the water). ↵
- Recall also that electronegativity stems directly from the effective nuclear charge on a particular atom. If you don’t remember why, go back to chapter 2 and review this important idea. ↵
- Although some highly-charged metal ions react with water, we will not consider these reactions at the moment. Group I and II cations are stable in water. ↵
- There are some nitrogenous compounds that are not basic because the lone pair is already being used for some other purpose. If you continue to study organic chemistry, you will learn about these ideas in more detail. ↵
- Note reactions between molecules are intermolecular reactions; those that involve a single molecule are intramolecular. ↵
- http://www.springerlink.com/content/n274g10812m30107/ ↵
- In fact Kw increases with temperature due to Le Chatelier’s principle, about which we will have more to say shortly. ↵
- The pH scale was first developed in 1909 by Danish biochemist Soren Sorensen. ↵
- In fact, pH is better defined as pH = {H3O+}, where the { } refer to the activity of the species rather than the concentration. This is a topic better left to subsequent courses, although it is important to remember that any resulting calculations on pH using concentrations provide only approximations. ↵
- Litmus is a water-soluble mixture of different dyes extracted from lichens, especially Roccella tinctoria— Wikipedia! ↵
- pH is typically measured by using a pH meter that measures the differences between the electrical potential of the solution relative to some reference. As the concentration of hydronium ion increases, the voltage (potential between the solution and the reference) changes and can be calibrated and reported as pH. ↵
- http://www.bbc.co.uk/dna/h2g2/A8819652 ↵
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1148806/pdf/biochemj00237-0011.pdf ↵
- This is based on the personal memories of one (and only one) of the authors. ↵
- Visit http://www.youtube.com/watch?v=eCk0lYB_8c0 for an entertaining video of what happens when sodium and other alkali metals are added to water (yes, they probably faked the cesium). ↵
- Hydrogen and oxygen can be used as rocket fuel, and the so-called “hydrogen economy” is based on the energy released when hydrogen reacts with the oxygen from the air. ↵
- http://www.youtube.com/watch?v=oXHtOjXxvRo ↵
- When O2 is used, the process is known as aerobic respiration. ↵
- Although bond dissociation energy and bond energy are often used interchangeably, they are slightly different. Bond dissociation energy is the energy required to break a particular bond in a molecule; bond energy is the average energy required to break a bond of that type. For our purposes, the difference is not important. Tables of bond energies usually refer to average bond energies. ↵
- Species with unpaired electrons ↵
- To begin this calculation, you must be able to figure out what bonds are present in the molecule; you must be able to draw the Lewis structure. ↵

