4 Heterogeneous Compounds
Melanie M. Cooper and Michael W. Klymkowsky
 Up until this point we have considered only bonds between atoms of the same element. While this makes things simpler (although you might not agree after thinking about the many forms of carbon), it leaves out the vast majority of the compounds that exist in the world and their chemistries. Moreover, pure elements are rare in nature. Much of the efforts of alchemists, early chemists, and the modern refining industry involve determining how to (economically) separate specific types of atoms (elements) away from others. Modern chemistry is concerned (largely) with putting atoms together to form new and useful molecules. Both involve understanding the concepts underlying how atoms interact.
Up until this point we have considered only bonds between atoms of the same element. While this makes things simpler (although you might not agree after thinking about the many forms of carbon), it leaves out the vast majority of the compounds that exist in the world and their chemistries. Moreover, pure elements are rare in nature. Much of the efforts of alchemists, early chemists, and the modern refining industry involve determining how to (economically) separate specific types of atoms (elements) away from others. Modern chemistry is concerned (largely) with putting atoms together to form new and useful molecules. Both involve understanding the concepts underlying how atoms interact.
4.1 3D and 2D Representations
To extend our discussion to the wider world of what we might call heterogenous molecules, that is, molecules made up of atoms of more than one element, we will begin with carbon. Why carbon? Well, here are some reasons. Carbon is the fourth most abundant element in the universe (~3,032 atoms per million), after hydrogen (~705,700 atoms per million), helium (~275,200 atoms per million), and oxygen (~5,920 atoms per million). Carbon is distinguished from most other elements in its ability to form a vast array of diverse compounds by bonding with itself and other elements with bonds that are not too strong and not too weak. Under the conditions that persist on the surface of the Earth carbon compounds are stable enough to hang around but not stable enough to persist forever, so they are not dead ends. Carbon is a key building block of the major molecules of life: proteins, nucleic acids, lipids, and carbohydrates. We are carbon-based life forms! Carbon compounds are also used in a wide range of synthetic materials, such as pharmaceuticals, polymers, and high-tech materials; we also consume a lot of carbon compounds by burning them for fuel.
Carbon: Always Tetravalent and Often Tetrahedral
Atoms combine in many different ways. We have already seen an example of how a covalent bond can form between two hydrogen atoms producing molecular (H2) as opposed to the atomic form of hydrogen. Similarly atoms of carbon can be linked together in various ways to form diamond, graphite, and graphene (see Chapter 3). Now we move on to molecules involving atoms of carbon and other elements. In keeping with our ongoing attempt to keep things simple (or better put, as simple as possible), let us start by examining the types of molecules that can be formed by combining carbon with hydrogen. There are many such molecules, and collectively they are known as hydrocarbons. The simplest such compound is methane CH4, a major component of natural gas. As in all its compounds and its elemental forms, carbon is tetravalent, which means that it always forms four bonds. We will now consider in greater detail why this is so, what forms the bonds can take, and what are the consequences of this fact. In this discussion, we will be building on the ideas introduced when we talked about diamond, graphite, and graphene.
To answer these questions we need to return to the ideas (introduced in Chapter 2) about the quantization of electron energy levels. Carbon has a total of six electrons, two of which are in a filled (1s) quantum shell, and four valence electrons; it is these valence electrons that can take part in bonding. Remember that the formation of a bond always lowers the energy of a system. It therefore makes sense that a carbon atom would form as many bonds as possible, resulting in the most stable possible molecular species.
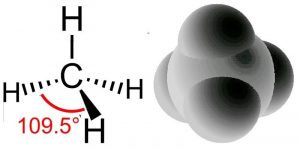
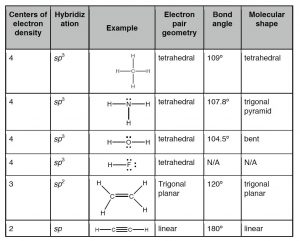
What happens if we combine hydrogen with carbon? Do we get a compound with properties intermediate between the two? Absolutely not, as you might have expected when considering the differences between diamonds and graphite. As previously we use the hybridization model to explain the behaviors we observe. We begin with what we know: in methane the carbon atoms make four bonds, one to each of four hydrogen atoms. We also know, from experiment, that the shape of the methane molecule is tetrahedral; there is a carbon at the center and the four C–H bonds pointing towards the corners of a four-sided figure. Since each C-H bond is formed from bonding orbitals we can use the model for bonding where these four bonding orbitals arise from the “hybridization” of the pre-existing 2s and three 2p atomic orbitals. The electrons in the 1s orbital are not used because the amount of energy needed to use those electrons is greater than the energy that would be released upon bond formation (they are held tightly to the nucleus by the electromagnetic force). It turns out to be a general rule that electrons in the core of the atom—in filled shells—tend not to take part in bonding. This means we need only consider the valence electrons when thinking about bonding.
The hybridization of the 2s and the three 2p orbitals results in four sp3 molecular orbitals, each of which can interact with the H atom’s 1s orbital to form a bond. When a bonding orbital is formed it contains two electrons. Because carbon has four valence electrons and each of the four hydrogens has one electron the result is a total of eight electrons distributed in four bonding orbitals.
Recall that we say the hybridization of carbon is sp3 and the arrangement of the bonds is tetrahedral, which means the angle between orbitals (and the C–H bonds) is 109.5º. Another way to say this is that the H–C–H bond angle is 109.5º. We can predict that this will be the case based on theoretical calculations; these have been confirmed by experimental observations. But why should this be true? How many different arrangements are there for four hydrogens bonded to a single carbon? Why aren’t the hydrogens all arranged in a single plane (around a central C with 90º bond angles) rather than in the tetrahedral arrangement? The planar arrangement, which is known as a square planar geometry, is actually possible and is sometimes observed under some special conditions, usually in molecules involving transition metals as we will see later). The square planar arrangement is not as stable as the tetrahedral arrangement for carbon because each C–H bond can be considered as a region of high electron (negative charge) density. Given that like repels like, each bond repels the others and moves as far away from the other bonds as possible. The optimum bond angle turns out to be 109.5º away from each of their neighbors. At that point, if they moved away from one orbital they would move closer to another. You may want to convince yourself of this geometric fact by using a marshmallow, toothpicks, and gumdrops! This principle goes by the unwieldy name of valence shell electron pair repulsion (VSEPR) and can be used to predict (once you get the hang of it) the three-dimensional (3D) structure of simple molecules—assuming that you know how the atoms within a molecule are connected. For example, using VSEPR logic, you should be able to present a compelling argument for why the C–H bonds in methane do not adopt a square planar orientation, as well as the general shape of many other types of molecules. You can even go further, in methane all four atoms attached to the central carbon are the same but what if they are different? You should be able to make plausible predictions about how bond angles would change if one of the attached groups is larger than the others – how would that influence bond angles?
One problem for many people is that 3D visualization of molecular structures is not easy. It is particularly tricky when one is called upon to translate the more or less abstract two-dimensional (2D) representations (Lewis and dot stuctures ↓) that you find printed on the page of a book, into a 3D model you can manipulate with your hands or in your mind. In addition, chemists (and molecular biologists) have an annoying tendency of representing complex 3D structures using various 2D representations, which can be confusing if you don’t know what you are looking at (or for). You have probably already seen some of these different structures, and we will consider a number of them below. Each provides specific kinds of information about the molecule. Note that actual 3D physical models and web activities can be very helpful in solidifying your ideas about structure.
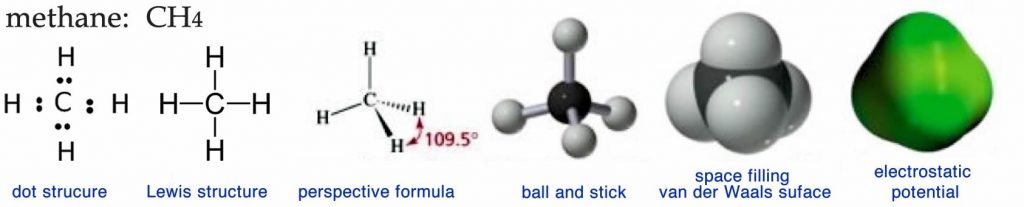
If we were able to see a methane molecule, what we observe would probably be closest to the electrostatic potential map. This visualization provides a picture of the surface of the molecule, generally color coded to represent fluctuations in electron density. Notice that there are no color fluctuations on this model of methane indicating that there are no (permanent) electron cloud distortions in the molecule—the surface of the molecule is uniformly electrically neutral. What is not so easy to discern from this representation is the fact that the methane is tetrahedral or that the central carbon atom is bonded to four hydrogen atoms, a fact that is much easier to appreciate in the other representations. The electrostatic potential representation is very useful for large biological molecules for several reasons: it is much simpler than the other kinds of models because individual atoms are not represented; it shows the molecule’s shape; and it shows where charges and partial charges are located.
The space-filling or van der Waals model gives more structural information in that the individual atoms that make up the molecule are distinguished by color (black for carbon, white for hydrogen, red for oxygen, and blue for nitrogen.) The surface of the model represents the molecule’s van der Waals radius, which is the distance where attraction turns to repulsion when two molecules approach one another. As its name implies, such models represent the space occupied by each atom.
The ball-and-stick model of methane shows the central carbon (black ball) attached to four hydrogens (white balls) by sticks that represent the bonds between the atoms. Although this model is probably the easiest to visualize, it is misleading because it could give the impression that bonds are like sticks holding the atoms together. It also does not represent either the actual volume occupied by the molecule or its electrostatic surface features. Another problem with all three of the preceding types of models is that you need a computer and specialized software (or some artistic ability) to draw them, which may not always be convenient or possible.
One strategy to address this problem is through what is known as a perspective formula. In a perspective formula the atoms are represented by their atomic symbols (for example, C or H) and bonds are represented by various kinds of lines. A normal line is meant to indicate a bond that is in the plane of the paper, a wedged line ![]() represents a bond that is coming out of the plane toward you (the reader), and a hatched line
represents a bond that is coming out of the plane toward you (the reader), and a hatched line ![]() represents a bond that is coming out of the plane, but away from you. This convention makes it easier to draw 3D perspective structures by hand without specialized software (or graphical talent.) We can, in fact, go one step further and draw methane without indicating its 3D structure at all. Structures that show all the bonds, atoms, and any valence electrons that are not in bonds, but do not attempt to accurately represent the 3D shape of a molecule are called Lewis structures. The Lewis structure for methane (see above) and the molecular formula CH4 represent a chemical shorthand that can provide a huge amount of information; we will see even more extreme examples as we go on. However, to be able to understand these representations, you must already know that the methane molecule is tetrahedral and the rules that apply to the geometry of carbon bonds, because neither is shown explicitly. If you didn’t know these things, you might even be tempted to assume that methane is organized with a square planar geometry or that the hydrogens are all located to one side of the carbon atom, neither of which is true!
represents a bond that is coming out of the plane, but away from you. This convention makes it easier to draw 3D perspective structures by hand without specialized software (or graphical talent.) We can, in fact, go one step further and draw methane without indicating its 3D structure at all. Structures that show all the bonds, atoms, and any valence electrons that are not in bonds, but do not attempt to accurately represent the 3D shape of a molecule are called Lewis structures. The Lewis structure for methane (see above) and the molecular formula CH4 represent a chemical shorthand that can provide a huge amount of information; we will see even more extreme examples as we go on. However, to be able to understand these representations, you must already know that the methane molecule is tetrahedral and the rules that apply to the geometry of carbon bonds, because neither is shown explicitly. If you didn’t know these things, you might even be tempted to assume that methane is organized with a square planar geometry or that the hydrogens are all located to one side of the carbon atom, neither of which is true!
Why, you might ask, would one want to draw structures with so much information missing? Perhaps, like medieval alchemists, modern chemists want to keep their secrets from the average person. Perhaps they just like secret codes and mystical symbols. Or perhaps it is because these shorthand representations of molecules are just much more compact and easy to draw, particularly when we get to large molecules with lots of atoms.[1] Drawing Lewis structures is an important and useful chemistry skill and we will return to it in more detail shortly. Once you have mastered it you will be able to look at a molecular formula such as CH4 (or C5H12) and (together with other information) be able to visualize the 3D structure of the molecule represented and predict many of the substance’s physical and chemical properties.
For example, models of the methane molecule predict that it is symmetrical. Again, this might not be entirely obvious just by looking at the structure, but if you make a model, or look at a rotatable interactive 3D model on the web you will see that it does not matter which way you look at the structure—all the C–H bonds are the same, and all the bond angles are the same. A little more information (which we will discuss later on) will let you deduce that there are no permanent electron density distortions in the molecule—just as is shown by the electrostatic potential map. Together these enable you to deduce that methane molecules are attracted to one another solely through London dispersion forces (like helium atoms or hydrogen molecules). Given how weak these interactions between molecules are we might be brave enough to predict that the melting and boiling points of methane are low (melting and boiling occur at relatively low temperatures) and we would be right! Methane melts at 91 K and boils at 112 K.[2]
Questions
Question to Answer
- Why (when present) are the four bonds formed by carbon usually arranged so that they point towards the corners of a tetrahedron?
Questions to Ponder
- If bond formation is stabilizing, why doesn’t carbon form six bonds, given that it has six electrons?
- Why doesn’t helium bond with carbon?
- What would be the consequences if carbon bonds with other atoms were very weak?
- What would be the consequences if carbon bonds with other atoms were very strong?
Building Increasingly Complex Molecules
You will soon realize that it is possible to build a rather amazing number of compounds using just hydrogen and carbon. For example imagine that we remove one hydrogen from a methane molecule; this leaves us with what is known as a methyl (-CH3) group. We can combine two methyl groups by forming a C–C bond between them (you might want to convince yourself that each carbon atom is still making four bonds with neighboring atoms). The resulting molecule is known as ethane (→). The structure of ethane can be written in a number of ways, for example H3C-CH3, CH3 –CH3 or C2H6. As the number of atoms increases so does the number of different ways a molecule can be represented. It is for this reason that chemists have developed a number of rules that are rather strictly adhered to; these rules make it possible to unambiguously communicate the structure of a molecule to others.[3] We will not spend much time on all of these various rules but there are web activities that you can do if you want to get an introduction and to practice them. These naming conventions are controlled by the International Union of Pure and Applied Chemistry, known as IUPAC and these rules can be found in the Compendium of Chemical Terminology.[4]
The process of removing hydrogens and adding methyl groups can continue, essentially without limit, to generate a family of hydrocarbons[5] known as the alkanes; the rules that govern these molecules are simple: each hydrogen makes one and only one bond; each carbon must make four discrete bonds; and these four bonds are tetrahedral in orientation. The number of carbons is in theory unlimited and how they are linked together determines the number of hydrogens. (Can you see how two hydrocarbons with the same number of carbon atoms could have different numbers of hydrogens?)
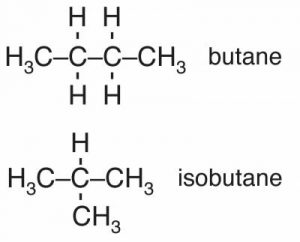 Depending on how the carbons are connected it is possible to generate a wide variety of molecules with dramatically different shapes. For example there are cage-like, spherical, and long, string-like alkanes. Consider the four-carbon alkanes. There are butane and isobutane that have the formula C4H10 as well as others with four carbons but different numbers of hydrogens, for example: cyclobutane, methylcyclopropane, and tetrahedrane. Butane has a boiling point of -0.5ºC, and isobutane has a boiling point of -11.7ºC. Why are the boiling points of butane and isobutane, which have the same atomic composition (C4H10), different? The answer lies in the fact that they have different shapes. The roughly linear carbon chain of butane has a larger surface area than isobutane, which gives it more surface area through which to interact with other molecules via London dispersion forces. This idea, that the shape of a molecule and its composition, determine the compound’s macroscopic properties is one that we will return to repeatedly.
Depending on how the carbons are connected it is possible to generate a wide variety of molecules with dramatically different shapes. For example there are cage-like, spherical, and long, string-like alkanes. Consider the four-carbon alkanes. There are butane and isobutane that have the formula C4H10 as well as others with four carbons but different numbers of hydrogens, for example: cyclobutane, methylcyclopropane, and tetrahedrane. Butane has a boiling point of -0.5ºC, and isobutane has a boiling point of -11.7ºC. Why are the boiling points of butane and isobutane, which have the same atomic composition (C4H10), different? The answer lies in the fact that they have different shapes. The roughly linear carbon chain of butane has a larger surface area than isobutane, which gives it more surface area through which to interact with other molecules via London dispersion forces. This idea, that the shape of a molecule and its composition, determine the compound’s macroscopic properties is one that we will return to repeatedly.
Questions
Question to Answer
- Why are the melting and boiling points of methane higher than the melting and boiling points of H2?
- How many different compounds can you draw for the formula C5H12?
- What structures could you imagine for hydrocarbons containing five carbon atoms?
- Is there a generic formula for an alkane containing n carbon atoms? How does forming a ring of carbons change your formula?
- Which has the higher boiling point, a spherical or a linear alkane?
- How do boiling points and melting points change as molecular weight increases?
Question to Ponder
- Make a prediction as to the melting and boiling points of ethane, compared to methane. What assumptions are you making? How would you test whether those assumptions are valid?
- Why does the shape of a molecule influence its behavior and its macroscopic properties?
4.2 Single Bonds and Molecular Shape
C–C and C–H bonds are described by molecular orbitals; calculations indicate that most of the electron density associated with these orbitals lies between the two nuclei. The C–H bonds have a length of 109 x 10-12 m (109 pm) while the C–C bond is approximately 50% longer, 154 x 10-12 m (154 pm). This is because the C–C bonding orbital is made from sp3 hybrid orbitals, which are larger than the 1s orbital that hydrogen uses to form bonds. These so-called σ (sigma) bonds have an interesting property; the atoms that they link can spin relative to each other without breaking the bond between them. For a C–H bond, if the H spins it would be impossible to tell, since the H atom is radially symmetric around the C–H bond axis. But if the carbons in the C–C bond of ethane spin relative to each other, then it is possible to observe different arrangements by looking down the C–C bond axis. For example:
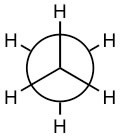 and
and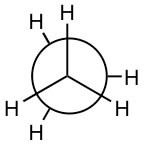 are both representations of ethane (the C–C bond is not seen in this depiction because you are looking straight down the C–C bond). They appear different because the arrangement of the atoms is different in space, but in fact at room temperature these two arrangements can easily interconvert by rotating around the C–C bond.
are both representations of ethane (the C–C bond is not seen in this depiction because you are looking straight down the C–C bond). They appear different because the arrangement of the atoms is different in space, but in fact at room temperature these two arrangements can easily interconvert by rotating around the C–C bond.
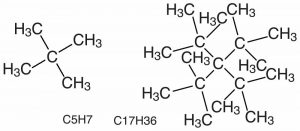
This raises another point to consider, namely that starting (and stopping) bond rotations requires energy. Similarly, there can be vibrations along the length of a bond, which again involves the absorption or release of energy. We will consider this further later on.[6] In the case of the rotating bond it turns out that as the bulk of the groups attached to the carbons increases the energy required for the rotation around the C–C bond also increases. Big, bulky groups can bump into each other, occupying each other’s space causing electron-electron repulsions and raising the energy of any shape where the groups are too close. This tends to lock the molecule into specific orientations that can influence the compound’s physical properties. An example of how structure interferes with the formation of a molecule is a molecule containing 17 carbon atoms and 36 hydrogen atoms (→); although it is possible to draw this molecule it has never been synthesized because the atoms crowd each other, and intrude on each other’s space. It is possible, however, to synthesize molecules with the same number of carbon atoms but fewer hydrogen atoms.[7] Can you produce a plausible explanation for why?
Collapsing Real Structures Down to 2-Dimensional Representations
Now, an obvious problem with complex three-dimensional molecules, even those made up only of hydrogen and carbon, is how to convey their structure when they must be depicted in two dimensions, like when you are writing on paper. Research indicates that students (that is, most people) have a tough time with this task, which is why we will describe various approaches here.
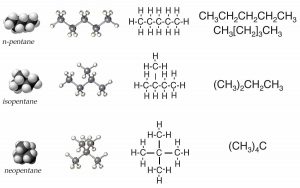 Before we begin, we need to have some rules. Let us use the set of possible molecules that contain 5 carbon atoms and 12 hydrogen atoms; these are generically known as pentanes. You can begin with a piece of paper and a pencil; how many different molecules can you draw with the composition of C5H12? Clearly C5H12 does not uniquely define the structure of the molecule; it is better to use their distinct names: pentane, isopentane, and neopentane (→). Each of the different molecules you have drawn has the same molecular formula but a different shape and, it turns out, different properties. For example, pentane has a boiling point of 308 K, whereas the boiling points of isopentane and neopentane are 301 K and 283 K, respectively. Their shape, rather than their elemental composition, influences the strength of the attractions between the individual molecules, which in turn influences their boiling points. We call these kinds of related compounds structural isomers, which means they have the same composition (for example C5H12) but their constituent atoms are connected differently to give different structures and shapes.
Before we begin, we need to have some rules. Let us use the set of possible molecules that contain 5 carbon atoms and 12 hydrogen atoms; these are generically known as pentanes. You can begin with a piece of paper and a pencil; how many different molecules can you draw with the composition of C5H12? Clearly C5H12 does not uniquely define the structure of the molecule; it is better to use their distinct names: pentane, isopentane, and neopentane (→). Each of the different molecules you have drawn has the same molecular formula but a different shape and, it turns out, different properties. For example, pentane has a boiling point of 308 K, whereas the boiling points of isopentane and neopentane are 301 K and 283 K, respectively. Their shape, rather than their elemental composition, influences the strength of the attractions between the individual molecules, which in turn influences their boiling points. We call these kinds of related compounds structural isomers, which means they have the same composition (for example C5H12) but their constituent atoms are connected differently to give different structures and shapes.
It is common to use a number of different types of representations to picture molecules. One way is through what are known as text formulas (or linear formulae). In this scheme, pentane is written CH3–CH2–CH2–CH2–CH3, which can also be written as CH3–[CH2]3–CH3. This captures some of the structural subtleties of pentane, but not all. For example, it does not illustrate the fact that the molecule is not strictly linear. Nevertheless, we can already anticipate complications. How would we write isopentane? The most obvious way would be (CH3)2CHCH2CH3. Neopentane is written as (CH3)4C. Does that make sense? Try deciphering them. We will return to this point later on in this chapter.
 If we followed the logic of this approach we could draw a more complete representation of pentane, isopentane, and neopentane as Lewis structures, but again, we are missing the three-dimensionality. You might even be led to think that the molecules are actually flat when they are much more like balls. Although it is possible to make the representation a little more realistic by trying to indicate three-dimensionality using the wedge and dash symbols, these structures become very complicated very fast. It is not really practical to draw out full 3D structures for larger, complex molecules. One important skill you will need to master is the ways that short-hand structures (such as Lewis structures) can provide information about the 3D structure of a molecule that allows us to predict chemical and physical properties.
If we followed the logic of this approach we could draw a more complete representation of pentane, isopentane, and neopentane as Lewis structures, but again, we are missing the three-dimensionality. You might even be led to think that the molecules are actually flat when they are much more like balls. Although it is possible to make the representation a little more realistic by trying to indicate three-dimensionality using the wedge and dash symbols, these structures become very complicated very fast. It is not really practical to draw out full 3D structures for larger, complex molecules. One important skill you will need to master is the ways that short-hand structures (such as Lewis structures) can provide information about the 3D structure of a molecule that allows us to predict chemical and physical properties.
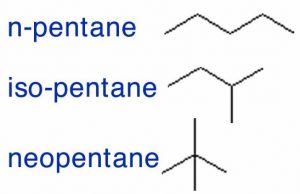
There is one more representation you will often see used that leaves out even more information. In the line structure the only things that are shown are the bonds between carbons! So for example for the pentanes (C5H12) we can draw structures such as those shown in the figure that omit all the symbols for atoms and all the C–H bonds. These structures should be used with caution because it is very easy to forget atoms or bonds when they are not in the representation. But what these line structures do show clearly is how the carbon atoms are connected, which can be very helpful at times.
Questions
Questions to Answer
- How many different compounds can you draw for C6H14? Draw out the full Lewis structure, the condensed formula, and the line formula.
- What are the advantages and disadvantages of each type of structure?
Questions for Later:
- When you think about rotating around a C–C bond (say in ethane), there are more and less stable orientations. Which orientation do you think is the most stable and why?
- Now imagine a butane molecule (C4H10) looking along the C2-3 bond. You would see one methyl group and two hydrogen atoms bonded to the two carbon atoms. How would that influence rotation around the C–C bond we have been considering?
4.3 Double and Triple Bonds
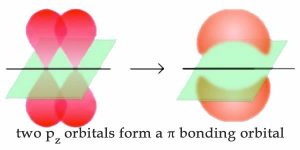 So far, we have considered what are known as single bonds; that is, all the C–C and C–H bonds in alkanes, and all the bonds in diamond. Each single bond involves two (and only two) electrons that are described by a bonding molecular orbital. In such a bonding orbital, most of the electron density is located between the two bonded atoms in a linear sigma (σ) bond. We have, however, already discussed albeit briefly bonds that involve more than one pair of electrons, namely those found in graphite. Recall that for graphite and graphene the bonds between carbon atoms in the sheet plane involve hybridized orbitals that are mixtures of the 2s2 and 2px and 2py (that is sp2 hybrid orbitals) leaving an unhybridized 2pz orbital. On bonding, these unhybridized 2pz orbitals reorganize to form what is known as a pi (π) bonding orbital. In π orbitals, the electron density lies above and below the axis connecting the bonded atoms. The combination of σ and π bonding orbitals produces a double bond. Double bonds are indicated by two lines, for example as in CH2=CH2 (ethene).
So far, we have considered what are known as single bonds; that is, all the C–C and C–H bonds in alkanes, and all the bonds in diamond. Each single bond involves two (and only two) electrons that are described by a bonding molecular orbital. In such a bonding orbital, most of the electron density is located between the two bonded atoms in a linear sigma (σ) bond. We have, however, already discussed albeit briefly bonds that involve more than one pair of electrons, namely those found in graphite. Recall that for graphite and graphene the bonds between carbon atoms in the sheet plane involve hybridized orbitals that are mixtures of the 2s2 and 2px and 2py (that is sp2 hybrid orbitals) leaving an unhybridized 2pz orbital. On bonding, these unhybridized 2pz orbitals reorganize to form what is known as a pi (π) bonding orbital. In π orbitals, the electron density lies above and below the axis connecting the bonded atoms. The combination of σ and π bonding orbitals produces a double bond. Double bonds are indicated by two lines, for example as in CH2=CH2 (ethene).
Shapes of Molecules with Double (and Triple) Bonds
 We can apply the same thinking about the arrangement of bonds around the carbon atoms in CH2=CH2 in much the same manner as we did for CH3-CH3. In ethene each carbon atom is surrounded by three centers of electron density, two Hs and one C. Note that the double bond counts as a single center of electron density (→). There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. First, a C=C double bond is typically less stable (that is more reactive) than two separate single bonds. When we come to thinking about reactions we will find that replacing a double bond by two single bonds typically produces a more stable system. Second, although there is more or less free rotation around the axis of a single bond at room temperature, rotation is blocked by the presence of a double bond. For a rotation to occur, the π bond (in which there is electron density above and below the axis between the two carbon atoms) must be broken and then reformed. The presence of a double bond has distinct effects on molecular shape. The minimum energy arrangement for three centers is a two-dimensional arrangement in which the groups are oriented at about 120º to one another; an arrangement known as trigonal planar geometry.
We can apply the same thinking about the arrangement of bonds around the carbon atoms in CH2=CH2 in much the same manner as we did for CH3-CH3. In ethene each carbon atom is surrounded by three centers of electron density, two Hs and one C. Note that the double bond counts as a single center of electron density (→). There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. First, a C=C double bond is typically less stable (that is more reactive) than two separate single bonds. When we come to thinking about reactions we will find that replacing a double bond by two single bonds typically produces a more stable system. Second, although there is more or less free rotation around the axis of a single bond at room temperature, rotation is blocked by the presence of a double bond. For a rotation to occur, the π bond (in which there is electron density above and below the axis between the two carbon atoms) must be broken and then reformed. The presence of a double bond has distinct effects on molecular shape. The minimum energy arrangement for three centers is a two-dimensional arrangement in which the groups are oriented at about 120º to one another; an arrangement known as trigonal planar geometry.
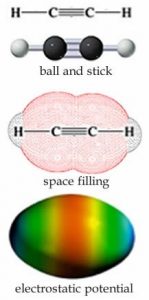 There is one more common type of bond that carbon can form, which is a triple bond. For example each carbon in C2H2 (ethyne) is surrounded by only two centers of electron density: a single sp hybrid orbital bonds between a carbon atom and a hydrogen atom and a triple bond, which can be thought of as a σ bond and two π bonds between the carbons, shown in the figure (→). The lowest energy arrangement around each carbon is a line in which the angle between the bonds is 180º. As before, a triple bond is less stable than three single bonds, and reactions can be expected!
There is one more common type of bond that carbon can form, which is a triple bond. For example each carbon in C2H2 (ethyne) is surrounded by only two centers of electron density: a single sp hybrid orbital bonds between a carbon atom and a hydrogen atom and a triple bond, which can be thought of as a σ bond and two π bonds between the carbons, shown in the figure (→). The lowest energy arrangement around each carbon is a line in which the angle between the bonds is 180º. As before, a triple bond is less stable than three single bonds, and reactions can be expected!
We see that under most conditions, a carbon atom can participate in a maximum of four bonds; either four single bonds, two single bonds and a double bond, or one single bond and a triple bond.
Questions
Questions to Answer
- Given a particular hydrocarbon, what factors would influence your prediction of its melting and boiling points? Can you generate some tentative rules?
- How does the presence of a double bond influence the structure of a hydrocarbon?
- How is the presence a triple bond different from that of a double bond?
- Why do you think there is no tetrabonded from of carbon (that is C four bonds C).
Questions to Ponder
- What limits the size and shape of a hydrocarbon?
4.4 Bonding in Nitrogen, Oxygen, and Fluorine
Even though the bonding of hydrogen and carbon atoms can generate a remarkable array of molecules, the hydrocarbons are really rather boring (chemically). They take part in a rather limited range of reactions and would not, on their own, be expected to produce anything like life. Of course there are many other elements, and their properties add chemical complexity to molecular behavior. From the perspective of living systems two of the most interesting elements are nitrogen and oxygen. Carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Nitrogen has seven electrons: two core and five valence: 1s2, 2s2, 2px1, 2py1, 2pz1. So if you are following the rules, you might well assume that nitrogen would be able to form five bonds (after all, it has five valence electrons). But when we look carefully, we never see a nitrogen atom making five bonds, and in all stable compounds it makes only three bonds. We can explain this observation in several ways. One factor is that nitrogen atoms are too small to support five centers of electron density around themselves because the bonds begin to overlap, which is destabilizing, just like we saw with bulky groups around a carbon. Another factor is that there are only four orbitals available in nitrogen in the second quantum shell. If nitrogen were to form five bonds it would have to use orbitals from the next quantum shell (3), but these orbitals are so high in energy that the energy required would not be offset by the energy released upon on bond formation. Together these factors mean that nitrogen, and in fact all elements in the second row of the periodic table, are limited to bonding arrangements with no more than four centers of electron density. As we will see later on, elements in the next row, such as phosphorus (P) and sulfur (S), are larger and have more available orbitals for bonding. These elements can form up to six centers of electron density.
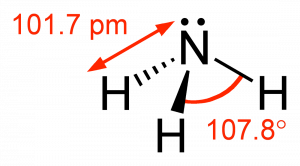
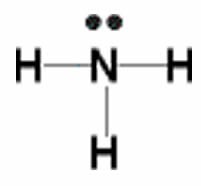
The simplest compound of nitrogen is molecular nitrogen, N2. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ and two π bonds. Molecular nitrogen, N2 is a stable (relatively nonreactive) molecular compound.[8]  A common nitrogen-containing molecule is ammonia (NH3), which is analogous to methane (CH3). In ammonia the nitrogen atom is bonded to three hydrogen atoms. These three bonds involve three of nitrogen’s valence electrons; the remaining two valence electrons occupy a non-bonding orbital and are referred to as a lone pair. Given the molecular hybridization orbital model that we are using this implies that four sp3 orbitals are formed from the nitrogen atom’s 2s and 2p orbitals leading to four electron density centers around the nitrogen. The figure shows three representations of ammonia. The first indicates the N–H bonds but fails to show the lone pair orbital. The second uses the wedge and dash convention and dots to illustrate the geometry of both bonds and the lone pair. The actual shape of the molecule is determined by the arrangements of electron clouds and the bonded atoms. In NH3 all three bonds are equivalent (N–H) and so must be symmetrical, but the lone pair orbital is different because it takes up more space than bonding pairs, can you imagine why? This has a subtle effect on the shape of the molecule. The angles between the C–H bonds in CH3 are equal and 109º while the angles between the N–H bonds in NH3 are slightly smaller, 107.8º. The shape of the molecule itself (as outlined by the atoms) is a triangle-based pyramid rather than a tetrahedron. Finally the Lewis structure (the most abstract representation), indicates the bonds and lone pair electrons but gives an unrealistic depiction of the molecule’s geometry. It is up to the reader to supply the implicit information contained in the structure about bond angles and overall shape.
A common nitrogen-containing molecule is ammonia (NH3), which is analogous to methane (CH3). In ammonia the nitrogen atom is bonded to three hydrogen atoms. These three bonds involve three of nitrogen’s valence electrons; the remaining two valence electrons occupy a non-bonding orbital and are referred to as a lone pair. Given the molecular hybridization orbital model that we are using this implies that four sp3 orbitals are formed from the nitrogen atom’s 2s and 2p orbitals leading to four electron density centers around the nitrogen. The figure shows three representations of ammonia. The first indicates the N–H bonds but fails to show the lone pair orbital. The second uses the wedge and dash convention and dots to illustrate the geometry of both bonds and the lone pair. The actual shape of the molecule is determined by the arrangements of electron clouds and the bonded atoms. In NH3 all three bonds are equivalent (N–H) and so must be symmetrical, but the lone pair orbital is different because it takes up more space than bonding pairs, can you imagine why? This has a subtle effect on the shape of the molecule. The angles between the C–H bonds in CH3 are equal and 109º while the angles between the N–H bonds in NH3 are slightly smaller, 107.8º. The shape of the molecule itself (as outlined by the atoms) is a triangle-based pyramid rather than a tetrahedron. Finally the Lewis structure (the most abstract representation), indicates the bonds and lone pair electrons but gives an unrealistic depiction of the molecule’s geometry. It is up to the reader to supply the implicit information contained in the structure about bond angles and overall shape.
Bonding of Oxygen and Fluorine
Let us now consider oxygen (O) which has eight electrons, two in the core and six valence (1s2, 2s2, 2px1, 2py1, 2pz1). As with nitrogen, oxygen does not use all its electrons to form six bonds because it is too small and the orbitals that would need to be used to make six bonds are too high in energy to be energetically accessible; that is, not enough energy would be released upon bond formation to “pay for” that energy.
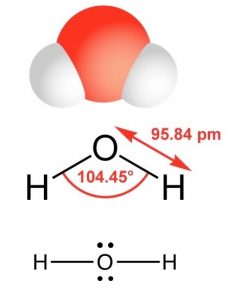 The simplest oxygen-containing molecule is molecular oxygen, O2. On our simple covalent bond model the two oxygen atoms are connected by a σ and a π bond, forming a double bond.[9] The next simplest, stable, most common, and by far the most important compound of oxygen at least from the perspective of living organisms, is water (H2O). In water there are two O–H bonds and two lone pair non-bonding orbitals. As in the case of nitrogen, the orbitals are sp3 hybrids and the oxygen atom is surrounded by four centers of electron density (see a pattern here?), two bonds, and two lone pairs. Again, the lone pair orbitals are larger than the O–H bonding orbitals, which distorts the tetrahedral symmetry of the molecule. Instead of equal angles of 109º between the orbitals, the angle between the O–H bonds is 104.5º. When we use a Lewis structure to represent the structure of H2O, it is critical to include all valence shell electrons.
The simplest oxygen-containing molecule is molecular oxygen, O2. On our simple covalent bond model the two oxygen atoms are connected by a σ and a π bond, forming a double bond.[9] The next simplest, stable, most common, and by far the most important compound of oxygen at least from the perspective of living organisms, is water (H2O). In water there are two O–H bonds and two lone pair non-bonding orbitals. As in the case of nitrogen, the orbitals are sp3 hybrids and the oxygen atom is surrounded by four centers of electron density (see a pattern here?), two bonds, and two lone pairs. Again, the lone pair orbitals are larger than the O–H bonding orbitals, which distorts the tetrahedral symmetry of the molecule. Instead of equal angles of 109º between the orbitals, the angle between the O–H bonds is 104.5º. When we use a Lewis structure to represent the structure of H2O, it is critical to include all valence shell electrons.
Continuing on across the periodic table we see that fluorine is the next element after oxygen. It has nine electrons: two core and seven valence. Rather than forming seven bonds fluorine only forms a single bond for basically the same reasons that oxygen only forms two bonds. Hydrogen fluoride, HF, has one bond, but four centers of electron density around the fluorine. Because HF has only two atoms, they must by definition lie on a line and therefore we do not need to discuss its shape.
| Compound | Molar mass
(g/mole) |
Boiling point | Bond type | Bond length (pm) | Atomic radius
(pm) |
| CH4 | 16 | –161 °C | C–H (in CH4) | 109 | C – 70 |
| NH3 | 17 | – 33 °C | N–H in (NH3) | 101 | N – 65 |
| H2O | 18 | 100 °C | O–H (in H2O) | 96 | O – 60 |
| HF | 20 | 19.5 °C | F–H in (HF) | 92 | F – 50 |
| Ne | 20 | –246.08°C | not applicable | not applicable | Ne – 38 |
 As we will see, a valid Lewis structure makes it possible to extrapolate a significant amount of information about a molecule’s chemical and physical properties. A confusing point is that the Lewis structure can be written in a number of apparently different ways, which are actually equivalent. The key to remember is that the Lewis structure does not attempt to depict a molecule’s actual three-dimensional structure. It is a shorthand (a “cartoon” if you like) that assumes you already know the arrangement of orbitals. No matter how it is drawn, the actual structure of a H2O molecule is the same with a 104.5º bond angle between the O–H bonds
As we will see, a valid Lewis structure makes it possible to extrapolate a significant amount of information about a molecule’s chemical and physical properties. A confusing point is that the Lewis structure can be written in a number of apparently different ways, which are actually equivalent. The key to remember is that the Lewis structure does not attempt to depict a molecule’s actual three-dimensional structure. It is a shorthand (a “cartoon” if you like) that assumes you already know the arrangement of orbitals. No matter how it is drawn, the actual structure of a H2O molecule is the same with a 104.5º bond angle between the O–H bonds
| CH4 | NH3 | H2O | HF | Ne |
| -258.7°F (-161.5°C) | -28.01°F (-33.34°C) | 212°F (100°C) | 67.1°F (19.5°C) | -410.9°F (-246.1°C) |
The tendency to form four centers (bonds or non-bonding pairs) has led to the rather misleading “octet rule”, which states that some elements tend to form molecules that have eight electrons around any atom (except for hydrogen). Unfortunately, the octet rule is far from being a rule because there are many exceptions, as we will see later. For example many of the elements past the second row of the periodic table are capable of bonding to more than four other atoms and some elements form stable compounds with less than eight electrons. It is important to remember that the octet rule is not the reason why atoms bond with each other, but it is a useful heuristic when constructing Lewis structures for the second row elements (C, N, O, F).
Polarized Bonds and Electronegativity
Earlier we saw that the boiling points of hydrocarbons tend to increase as the number of carbons in the compound increases and that molecules with similar molecular weights have similar but not identical boiling points, with the shapes of the molecules having an effect, although a relatively small one. The attractions between hydrocarbons are due to London dispersion forces that depend on the size, surface area, and shape of the molecule. The larger these forces, the more strongly molecules will stick together and the more energy (higher temperature) will be needed to overcome these attractions.
Let us consider the boiling points of some common second row compounds involving bonds with hydrogen, that is, CH4, NH3, H2O and HF, and neon (Ne), which does not form bonds with hydrogen (the compounds of lithium, beryllium, and boron with hydrogen are much less common.) These compounds all have about the same molecular weight but different shapes. Based on our experiences with hydrocarbons, we would be well justified in predicting that they would have somewhat similar boiling points. Unfortunately, this prediction is not supported by experimental evidence (see Table). There is no clear trend, so something is going on that we have not yet considered. To explain this data we have to return to an idea that we discussed in Chapter 3, namely that the size of atoms decreases as you go across a row of the periodic table. Not only does the size (radius) of the atoms decrease (from 70 pm for carbon to 38 pm for neon) but so does the length of the bonds between the atoms and hydrogen (from 109 pm to 92 pm). This is both surprising and counterintuitive (which is why we are reminding you about it!)
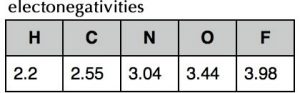
Remember that the size of the atom is based on a balance between the attraction between the negatively charged electrons to the positively charged protons in the nucleus, the repulsions between the electrons as they get close to each other, and of course the arcane, but highly accurate rules of quantum mechanics. The reason that the atom’s size is decreasing as the number of protons increases is that each electron in the valence shell is attracted by an increasing number of protons in the nucleus. The more protons, the larger this attractive force. At the same time, the electrons in the same valence shell do not tend to repel each other as much as you might suspect because they are in different orbitals. Therefore the effective nuclear charge increases from left to right across the periodic table. This increase in effective nuclear charge doesn’t just affect the electrons in isolated atoms; it also affects the electrons in bonds. The ability to attract the electrons in bonds is called electronegativity, and because it derives from the same effect as that that determines effective nuclear charge and atomic radius, electronegativity also tends to increase from left to right across a row in the periodic table. It also decreases from top to bottom in a group of the periodic table. This makes sense because the further electrons are from the nucleus, the less they will be attracted to it. The exceptions to this rule are the noble gases (helium, neon, argon, etc.); because they do not form bonds with other elements (under normal circumstances) their electronegativities are usually not reported.
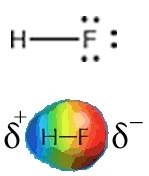
Fluorine is the most electronegative element and the Lewis structure of HF shows one H–F bond and three lone pairs. Fluorine attracts electrons very strongly—even the ones in the H–F bond so that the fluorine atom ends up with more than its fair share of electrons and the hydrogen atom ends up with less. One way to think about this is that the electron density in the H–F bond is shifted closer to the fluorine atom and away from the hydrogen atom (→). The result of this is that the fluorine atom has more negative charge than positive charge and the hydrogen atom has more positive than negative charge. We indicate this by writing a δ– charge on the fluorine atom and a δ+ charge on the hydrogen atom (δ is often used to denote a small increment, that is less than 1). That means that there is an unequal distribution of charge in the molecule. The HF molecule has a permanent dipole, that is, a separation of charge; the H–F bond is said to be polarized and the molecule is considered polar. Permanent dipoles are different from the transient dipoles associated with London dispersion forces. Because of their permanent dipoles molecules of HF interact with one another both attractively and repulsively, more strongly in some orientations than in others. HF molecules are attracted to each other much more strongly than neon atoms, for example, because of the presence of these permanent dipoles. This results in a much higher boiling point for HF than for neon (see above). That is, much more energy has to be supplied to the system to overcome the force of attraction and to separate HF molecules from each other than is needed to separate neon atoms. An important point to note is that HF only has one bond, and the polarity of the bond is the same as the polarity of the whole molecule. As we will see, this is not the case in molecules with more complex structures.
It is relatively easy to predict whether a particular bond is polar by looking at the electronegativity differences between the atoms in that bond. Typically, elements on the left-hand side of the periodic table (metals) have rather low electronegativities and elements over toward the right-hand side (non-metals) have higher electronegativities. There are several ways to calculate electronegativities but in general it is not very useful to memorize specific numbers. It is helpful, however, to understand the trends and to be able to predict bond polarities. Because fluorine is the most electronegative element it can be expected to make the most polarized bonds with hydrogen.[10] So let us take this logic a bit further. If HF has the most polar bonds then HF molecules should stick together with the strongest attractions and HF should have the highest boiling point. But oh no! Water’s boiling point is significantly higher (100 ºC compared to 19 ºC for HF). What is going on? Oxygen is not as electronegative as fluorine and so the O–H bond is not as polar as the H–F bond. Why then is the boiling point of H2O 81 °C higher than HF? To answer this question we need to consider another factor that affects the polarity of a molecule – and that is molecular shape.
Questions
Questions to Answer
- Why do you think that the trends in effective nuclear charge, ionization energy, and electronegativity are correlated?
- What does correlated mean?
- Can you draw a picture of (say) four H–F molecules sticking together?
- Is there any arrangement that they might take up or would they stick together in a totally random way?
Questions to Ponder
- Why would you not expect polymeric oxygen, that is molecules similar to hydrocarbon chains (or perhaps you would)?
4.5 Molecular Shapes, Polarity, and Molecular Interactions
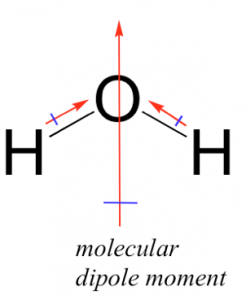
Now we really have to begin to use our 3D thinking and consider several additional factors: the shape of the molecules and how they interact. Much of this thinking is best done hands on with molecular models but we will outline the logic involved here. The HF molecule has a simple shape; it is linear with (partially) positively and (partially) negatively charged ends. In contrast, the H2O molecule has a more complex shape; it has two polar O–H bonds. To understand how this affects the polarity of the molecule we have to take into account the number of bonds, their polarization, and the overall shape of the molecule. Bond polarity is a vector quantity, which means it has both a magnitude and a direction. This is where an understanding of the 3D structure of the molecule becomes critical. Each O–H bond is polarized and the overall polarity of the molecule is determined by the vector sum of these bond polarities (that is you have to take into account both the magnitude and the direction of the bond dipoles). This may sound a bit complicated but in practice it is relatively easy to predict qualitatively what the overall polarity of the molecule is as long as you keep in mind its 3D structure. In water the two O–H bonds are at an angle of about 107° to each other (→). If we add the bond dipole moments up you can see that the overall direction of the dipole for the molecule bisects that angle, as shown in the figure. Now you might think that this exercise is a bit of a waste of time—surely it would make sense that if a molecule has polar bonds, then the molecule itself should be polar. However, as we will see shortly this is not always the case.
If we apply a similar analysis to ammonia (CH4) we see that the N–H bond is polar with a δ+s on the hydrogen atoms and a δ– on the nitrogen atom. Remembering that the actual shape of NH3 is a triangular based pyramid, with an H–N–H bond angle of ~105º, we can see that there is an overall dipole moment in ammonia. Therefore ammonia is a polar molecule.
If we contrast this with methane, however, we see two differences. The first is that carbon is not nearly as electronegative as nitrogen, oxygen, or fluorine, so the C–H bond is not as polar. That said, there is an electronegativity difference and so the electron density in the C–H bond is distorted towards the carbon atom (because it is a little more electronegative than the hydrogen atom.) At the same time, CH4 is symmetrical (tetrahedral.) If we add up all the bond dipoles they cancel each other out giving a molecular dipole moment of zero. Even if we were to replace the hydrogen atoms in methane with fluorine atoms to give CF4 (carbon tetrafluoride) the resulting molecule would still be non-polar, despite the fact that the electronegativity difference between carbon and fluorine is greater than that between hydrogen and oxygen! This is another example of something counterintuitive: something made up of polar parts that is not polar.
The Famous Hydrogen “Bond”
Now that we have a better idea of how the shape and types of bonds in a molecule can affect its polarity, let us look a little more closely at how molecules interact with each other. The first thing to note is that globally non-polar molecules interact solely via London dispersion forces just like atoms of neon or helium. The boiling point of neon is –246 °C while the boiling point of CH4 is –161 °C. This means that methane molecules are more strongly attracted to each other than are neon atoms. We can explain this based on the fact that a methane molecule is larger than a neon atom. Because the electrons in methane molecules are dispersed over a larger area and their distribution (in space) is easier to distort, we say methane molecules are more polarizable. At the same time because methane molecules are non-polar, the boiling point of methane is much lower than that of substances made of polar molecules of similar size.[11]
Let us consider three such molecules: HF (bp 19.5 °C), H2O (bp 100 °C), and NH3 (bp -33 °C). All three are polar so they stick together but why are there such large differences in their boiling points? The answer lies in the fact that the molecules interact with one another in multiple ways. They all interact via London dispersion forces and dipole–dipole interactions. In addition, a new type of interaction, known as a hydrogen bond (or H-bond) is also possible. The term H-bond is somewhat misleading because these are much weaker than covalent bonds and do not involve shared electrons; the energy required to break a typical hydrogen bond is between 5 and 30 kJ/mole, whereas it requires over 400 kJ/mole to break a C–C bond.[12] In biological systems and in liquid water, H-bonds are continuously breaking and reforming. Hydrogen bonds are formed between two separate molecules.[13] In contrast to London dispersion forces, but like covalent bonds, H-bonds have a direction; they form when the hydrogen of one molecule, which is covalently bonded to an O, N or F, is attracted by the lone pair on an O, N of F of a neighboring molecule.
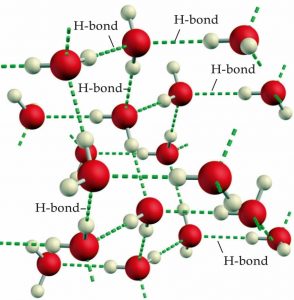
H-bonds are a special case of an electrostatic interaction involving a hydrogen atom that is bonded to a very electronegative atom (typically oxygen or fluorine) and an electronegative atom that has lone pairs of electrons. When a hydrogen is bonded in this way most of the electron density moves toward the electronegative atom, leaving a relatively large δ+ on the hydrogen. Water is a particularly important example of a molecule able to engage in hydrogen bonding, because each molecule of water has the possibility of forming four H-bonds (→). Each of the hydrogen atoms within a water molecule can bond to another water molecule, while each oxygen atom has two lone pairs that can interact with the electron-deficient hydrogen atoms of two different neighboring water molecules, shown in the figure. The ability to form large numbers and networks of hydrogen bonds is responsible for many of the unique properties of water including its relatively high melting point, boiling point, heat capacity, viscosity, and low vapor pressure. In contrast, HF and NH3 can form, on average, only two H-bonds per molecule. Can you figure out why this is so? Because there are fewer H-bonds to break, they have lower boiling points. HF has a higher boiling point than NH3 because the H-bonds in HF are stronger than those in NH3. (Can you figure out why?) In addition to their role in the bulk properties of substances like water, we will see that H-bonds play a critical role in the organization of biological systems, from the structure of DNA and proteins, to the organization of lipid membranes and catalytic mechanisms (but more about that later).
Other Polar Bonds
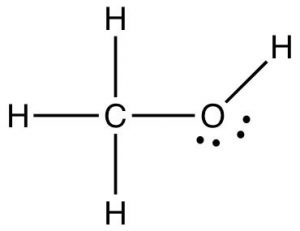
We have seen that when hydrogen is covalently bonded to oxygen, nitrogen, or fluorine, the result is that the covalent bond is highly polarized and the majority of the electron density is located on the most electronegative atom. This means that the hydrogen atom has very little electron density remaining around it. Because hydrogen is such a small atom, the resulting positive charge density on the hydrogen atom is high. This leads to unusually strong attractions (H-bonds) with atoms that have lone pairs with which the positively charged hydrogen atom can interact. H-bonding is unique to molecules in which a hydrogen atom is covalently bonded to an oxygen, nitrogen, or fluorine atom. However, there are uneven charge distributions possible whenever two atoms with different electronegativities form a bond. Consider, for example, methanol (CH3OH). It has several different types of bonds with different distributions of charge in them. The familiar O–H bond in methanol is very much like the O–H bond found in water. That is, it is highly polarized and the hydrogen atom is a small, dense region of highly positive charge that can attract and will be attracted to regions of high electron density such as the lone pairs on oxygen. The methanol molecule also has a C–O bond and three C–H bonds. If we consider the differences in electronegativity we can predict the polarization of these bonds. Remember that carbon and hydrogen have quite similar electronegativities, and so the C–H bond is not very polarized. Carbon and oxygen, in contrast, are quite different in their electronegativities and the result is that the C–O bond is strongly polarized, with the δ+ located on the carbon atom and the negative end of the bond dipole on the oxygen atom. As we will see later this has implications for how methanol (and all C–O containing compounds) interact (and react) with other substances.
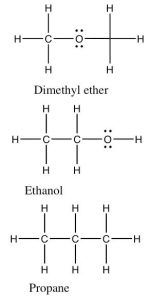
An inspection of the Lewis structure can reveal (to the trained mind!) a huge amount about the structure and polarity of a molecule and taking that one step further we can make predictions about the properties of the compound. For example if we compare the relative boiling points of methanol (CH3OH, bp 65 °C) and ethane (CH3CH3, bp -88.6 °C) we see (just as you already predicted no doubt) that methanol has a much higher boiling point because it takes more energy to separate molecules of methanol. The question arises: is this because methanol can form an H-bond with itself? Can you draw a picture of how this happens? Or is it because of the C–O dipole? We can look at this idea a little more closely by comparing the boiling points of three compounds that have similar molecular weights (so that they experience similar London dispersion forces), but different types of bonds in them.
If we classify the kinds of bonds as before we see that dimethyl ether has non-polar C–H bonds and polar C–O bonds. The C–O–C bond angle is about 104°. Because each atom (except for H) is surrounded by four centers of electron density, the molecule is not linear as pictured. (Why not?) The molecule as whole is polar but cannot form hydrogen bonds with itself because none of the hydrogen atoms have a significant δ+ as they would if they were bonded to an oxygen atom. We call the type of forces between dimethyl ether molecules, dipole–dipole forces. On the other hand, an ethanol molecule—which has exactly the same molecular weight and formula—can form hydrogen bonds with itself because it has an O–H bond, and so has a small partially positively charged hydrogen atom. This minor difference has a huge effect on boiling point: ethanol boils at 78 °C whereas dimethyl ether boils at –23 °C. Both of them are considerably higher than propane at -44 °C (remembering that absolute zero is –273.15 °C). From comparing these three similar compounds we can see that a simple dipole–dipole attraction increases the boiling point by 21 °C, and on top of that the H-bonding attraction in ethanol is worth another 99 °C, bringing the boiling point of ethanol to 78 °C.
Intermolecular Forces
Taken together, London dispersion forces, dipole-dipole interactions, and hydrogen bonds comprise a set of attractive forces that make separate molecules stick together. These are collectively named intermolecular forces, IMFs. These forces are caused by either permanent or temporary distortions of the electron cloud in a molecule – which leads to electrostatic attractions between separate molecules. For small molecules, the typical order for strengths of IMFs is:
H-bonding (where available) > dipole–dipole interactions > London dispersion forces. At the same time, because London dispersion forces increase with molecular size and the extent of surface-surface interactions, they are often the predominant intermolecular force between large biological macromolecules.
The Importance of Shape
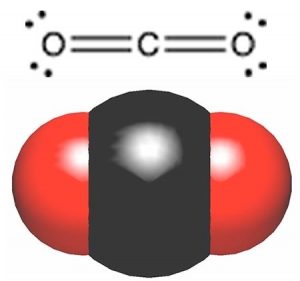
While we are on the subject of carbon and oxygen containing compounds, let us take a look at one of the most common compounds of carbon and oxygen, carbon dioxide. You can draw the structure of CO2 with the carbon atom in the middle, double bonded to each of the oxygen atoms. That is, CO2 has two quite polar bonds in it, and so we might reasonably predict that its boiling point might lie somewhere between dimethyl ether and ethanol. But, as you probably already know, this is not the case. CO2 exists as a gas at room temperature. In fact CO2 does not have a liquid phase at standard atmospheric pressure; it changes directly from a solid to a gas, a process called sublimation, at -78 °C. How is such behavior to be understood, particularly given that CO2 has about the same molar mass as ethanol (CH3CH2OH), which is a liquid at room temperature? Once again we have to make sure we have considered all the factors that affect molecular polarity including bond polarity and shape. If you reflect back to the ideas about bond polarity and structure you will see that we have another case here of a molecule with polar bonds, but no overall polarity. CO2 has a linear structure so the bond polarities cancel each other out (they are at 180º from each other) (→). CO2 has no overall molecular polarity, even though it has polar bonds. Therefore the molecules do not stick together very well and it is a gas at room temperature.
Questions
Questions to Answer
- What is the direction of the molecular dipole moment in ammonia? Draw out a picture showing how you came up with the answer. Does it matter which way you draw the molecule? What if you draw it upside down? Will that affect the direction of the dipole (in the real world)?
- Why are the interactions between H2O molecules stronger than those between HF molecules even though the polarity of the HF bond is larger than the polarity of the OH bond?
- Why don’t more than four water molecules interact with a central water molecule?
- What would you predict would be the relative boiling points of methanol (CH3OH) and ethane (CH3CH3), which have similar molecular weights?
- What would you predict would be the relative boiling points of methanol (CH3OH) and ethanol (CH3CH2OH)?
- What kind of compound (or what structural feature) would you expect might be attracted to the δ+ located on the carbon atom in methanol?
Questions to Ponder
- What would be the consequences (for life, the universe, and everything) if water molecules were linear?
4.6 Ionic Bonding
Our discussion up to now has centered on types of bonds that involve valence electrons being shared between (or more correctly being fought over – attracted to the opposite nuclei) different atoms. We have seen that we can consider electron density to be equally distributed between the bonding atoms, or that it may be distorted by being attracted to the more electronegative atom. What we have not looked at yet is the extreme case of this kind of distortion, in which the valence electrons are attracted so much by the electronegative atom that they are transferred completely. This kind of bonding is called ionic bonding (as you are almost certainly already aware).
Let us take a look at some common ionic compounds and see if we can make some sense of their properties from a consideration of their atomic-molecular structure. For the sake of simplicity we will confine ourselves (for the moment) to binary compounds (compounds with only two elements in them.) The most familiar of these compounds is sodium chloride (NaCl), common table salt. NaCl is a continuous compound that extends in three-dimensional array much like diamond (see Chapter 3.) NaCl is a solid at room temperature, with a very high melting point (801 °C), similar to the melting points of silver (961.78 °C) and gold (1064.18 °C), although much lower than the decomposition temperature of diamond (3550 °C). An interesting difference between diamond and sodium chloride occurs on heating. Remember diamond does not melt; it decomposes once enough energy is added to the system to break the C–C bonds. Under normal circumstances, the carbon atoms react with oxygen (O2) in the air to form carbon dioxide—a process that requires the addition of lots of energy to reverse (as we will see later). On the other hand NaCl melts (solid → liquid) and freezes (liquid → solid) at 801 °C, much like water, just at a higher temperature. Based on this difference, we might be tempted to conclude that covalent bonds are not broken when salt melts but that something stronger than the H-bonds that hold water molecules together are broken. What could that be?
A hint comes from studies first carried out by the English chemist Humphrey Davy.[14] Davy used a voltaic pile to study the effects of passing electricity through a range of substances.[15] Solid table salt did not conduct electricity, but liquid (molten) salt did. Not only did it conduct electricity, but when electricity (electrons) was passed through it, it decomposed to produce globules of a shiny, highly reactive metal (sodium, Na) and a pale green gas (chlorine, Cl2). Davy correctly (as it turned out) deduced that the elements in table salt (what we now know as sodium and chlorine) are held together by what he termed electrical forces. Just what caused those electrical forces was not discovered until the atomic nature of matter was elucidated over 100 years later.
It takes a great deal of energy to change table salt into its constituent elements. First the salt has to be heated to its melting point, and then electrical energy must be added to release the elements sodium and chlorine. The reverse reaction, combining the elements sodium and chlorine (don’t do this at home), produces sodium chloride and releases a great deal of energy (411 kJ/mol). Given the release of energy, we suspect that bonds are being formed during this reaction.
One of the important principles of chemistry is that structure on the atomic-molecular level is reflected in the behavior of materials in the real world. So, let us review some of the real-world properties of sodium chloride:
- It forms colorless crystals that are often cubical in shape and are hard and brittle.
- It has a high melting point and conducts electricity when melted, but not in the solid state.
Based on these properties, and what we know about interactions, bonds, and electricity, we can begin to make hypotheses about how atoms are organized in NaCl. For example, the fact that NaCl is a stable, crystalline solid at room temperature and that it melts at a high temperature implies that forces holding the atoms together are strong. The regular shape of salt crystals implies that bonds holding the atoms together extend in three dimensions with some regular pattern. If you take a large salt crystal and give it a sharp knock, it breaks cleanly along a flat surface. Diamond also behaves in this way. The ability of molten, but not solid, salt to conduct electricity suggests that melting leads to the appearance of moveable, electrically charged particles. The current interpretation of all these observations and experiments is that in the solid state salt (NaCl) is held together by the coulombic (electrical) attractions between sodium (Na+) and chloride (Cl–) ions. So when sodium metal (Na) reacts with chlorine (Cl2) gas, sodium and chloride ions are produced. In the solid state, these ions are strongly attracted to each other and cannot move, but they can move in the molten (liquid) state, and their movement is what conducts electricity (electrons).
One way to think of ionic bonding is that it is the extreme limit of a polar covalent bond. Typically, simple ionic compounds are formed from elements on the left-hand side of the periodic table (metals, such as sodium) and elements on the right-hand side (non-metals, such as chlorine). The non-metals tend to have a high electronegativity as a result of their high effective nuclear charge, whereas the metals have low electronegativity because their valence electrons are not very strongly attracted to their nuclei. When a metal atom meets a non-metal atom the non-metal attracts the valence electrons from the metal, so that for all intents and purposes electrons move from the metal atom (which then has a net positive charge) to the non-metal atom (which now has a net negative charge). This effect, however, applies only to the electrons in the unfilled valence shells. Electrons in a metal atom’s filled core orbitals require a lot more energy to remove. Why? Because they are closer to the positively charged nucleus (recall the jump in ionization energy when an electron is removed from the core). If there is a single outer-shell electron (as is the case with Na and other Group I metals) that electron is often lost, and the resulting atom (now called an ion) has a single positive charge (for example, Na+). If there are two outer-shell electrons, as in the case of the Group II metals, such as calcium and magnesium, both can be lost to produce doubly charged ions, such as Ca++ and Mg++ (usually written as Ca2+ and Mg2+). At the other side of the periodic table, the non-metals show exactly the opposite pattern, gaining electrons to become negatively charged ions.[16]
Questions
Questions to Answer
- The melting point of table salt is over 800 °C. Why is it so high?
- What properties do you associate with a solid?
- What happens on the atomic-molecular level when a solid melts?
- Why don’t metals tend to gain electrons? Why don’t non-metals lose electrons?
- What happens to the size of a sodium atom when it loses an electron to become Na+?
- What happens to the size of a chlorine atom when it gains an electron and becomes Cl–?
Questions to Ponder
- Why doesn’t solid table salt conduct electricity?
- Why does molten table salt conduct electricity?
Back to Sodium Chloride
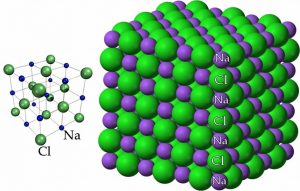
By this point, we have concluded that NaCl is composed of Na+ ions (cations) and Cl– ions (anions), but we have not yet discussed how these ions are arranged with respect to one another in space. As you may have come to expect, there is usually more than one way to represent a chemical structure. Different models emphasize different features of a substance but none of them are real in the sense that if we could look at the molecular-level structure, these models are not what we would see. At the same time, visible cubes of salt crystals provide a clue to atomic-molecular structure. If we follow the structure down from the macroscopic to the molecular, this cubic/rectangular structure is retained. A diagram of sodium chloride showing the relative positions of the ions, shown here, illustrates this cubic organization.
Another way to look at NaCl is to think of each Na+ ion as being surrounded by six Cl– ions, and each Cl– ion is surrounded by six Na+ ions. Such an arrangement is possible because of the relative sizes of the sodium and chloride ions; the smaller Na+ ions can sit in the holes between the larger Cl– ions (why are the chloride ions bigger than the sodium ions?). One consequence of this arrangement is that there is not an “ionic” bond that is analogous to a covalent bond. Our model of bonding here is best understood as this three-dimensional lattice of interacting ions. The alternating network of positive and negative ions makes for a very stable structure that is difficult to disrupt. The implication? Lots of energy is required to break these interactions and allow the ions to move with respect to one another. Many ionic compounds are organized in similar kinds of crystalline structures. A complexity (to which we will return in Chapter 6) is that many ionic compounds, including NaCl, are highly soluble in water, which means they interact strongly with water molecules. Often salts crystallize together with water molecules and form hydrated (with water) forms, as opposed to anhydrous (without water) forms.
How Ionic Bonding Explains the Properties of Ionic Compounds
Let us return to the properties of ionic compounds and see how this molecular-level (microscopic) model of bonding explains their properties. First, their high melting points arise from the fact that enough energy must be supplied so that multiple (strong) coulombic interactions (recall each cation is surrounded by six anions and vice versa) between the ions must be overcome. In contrast for water, it is only the intermolecular forces between molecules that must be overcome to melt ice; IMFs are significantly weaker than full ionic interactions. Similarly it takes even more energy to vaporize (liquid → gas) NaCl.
Now let us predict the melting points of different ionic compounds. Remember that the force between the ions is a Coulombic attraction: F α (q+ x q– ) / r2, where q+ and q– are the charges on the ions, and r is the distance between them. This equation tells us that as the charge on the ions increases, so does the force of attraction, but as the distance between them increases, the force of attraction decreases. That is, the coulombic attraction should be larger for small, highly charged ions, and this should be reflected in the melting points of ionic compounds. Even when we don’t factor in the size of the ions, q1 x q2 = 4 which means that the attractive forces for CaO should be on the order of 4 times those for NaCl. Indeed, the melting point of calcium oxide (CaO) which has q1 = 2+ and q2 = 2– is 2,572°C.
Questions
Questions to Answer
-
- Draw a molecular level picture of liquid water, and a molecular level picture of liquid sodium chloride. Use this picture to explain why it takes more energy to melt solid salt than it does to melt solid water.
- Arrange these ionic compounds in order of increasing melting point: NaCl, KBr, CaO, Al2O3. Look up your answers and see if your predictions were correct.
- Arrange these materials in order of increasing melting point: CH4, MgBr2, HF, C(diamond). Look up your answers and see if your predictions were correct.
- What do you think happens to the size of the particle when a chlorine atom gains an electron to become a chloride ion? (hint recall that the size of an atom depends on the balance between the attractions between the electrons and the nucleus, and the repulsions between the electrons)
- What do you think happens to the size of the particle when a sodium atom loses an electron to become a sodium ion?
- We do not believe that their intent is to torment students, and perhaps they have just forgotten that not every student knows or remembers all of the rules. ↵
- Unless otherwise noted, we always consider melting and boiling points at atmospheric pressure. ↵
- Chemists are not being unnecessarily difficult; anatomists also have a very strict set of names for the various bones and nerves in the body, in part to avoid confusion during medical procedures. ↵
- http://www.iupac.org/ and http://goldbook.iupac.org/index.html ↵
- Hydrocarbons contain only hydrogen and carbon. Be careful not to confuse them with carbohydrates, which contain carbon, hydrogen, and oxygen and include sugars. We will consider carbohydrates in more detail later on. ↵
- In fact, we will see that these rotations and vibrations are quantized! ↵
- http://pubs.acs.org/doi/full/10.1021/ci0497657 ↵
- However, a nitrogen compound with some structural similarities to diamond has been identified. It was synthesized from N2 at high pressure and temperatures. In this polymeric nitrogen, each nitrogen is connected to three neighbors via single bonds, in a similar way that diamond has carbons connected to four neighbors. However this polymeric nitrogen is highly unstable and reactive – unlike diamond. http://www.nature.com/nmat/journal/v3/n8/abs/nmat1146.html ↵
- Interestingly O2 cannot be well described by a simple valence bond model, because it can be shown that molecular oxygen has two unpaired electrons (it is a di-radical). The bonding is best explained by using molecular orbital theory. ↵
- Another way to talk about polarity is to say the bond (or molecule) has a dipole moment (unit Debye)- that is the magnitude of the charges x distance separating them. ↵
- It is worth keeping in mind the distinction between the molecules a substance is composed of, and the substance itself. Molecules do not have a boiling point, substances do. ↵
- Remember what a mole is, and that a kilojoule (kJ) is a unit of energy. ↵
- In larger molecules, such as proteins and nucleic acids, H-bonds can also form between distinct regions of a single molecule. ↵
- While Davy is well known now for his experiments on the nature of salts, he began his chemical career in his early twenties researching medical uses of gases. He apparently became very fond of nitrous oxide (N2O, laughing gas), which he reported was an enjoyable recreational drug and a cure for hangovers (ref SALT). ↵
- In 1800 the first electric battery, the Voltaic Pile, was developed. It was promptly put to use by a growing number of scientists. For example, molecular hydrogen and oxygen could be produced by passing electricity through water. ↵
- Positively charged ions are known as cations and negatively charged ions are known as anions. ↵

