6 Solutions
Melanie M. Cooper and Michael W. Klymkowsky
We have covered quite a number of topics up to this point: the structure of atoms, discrete molecules, complex network solids, and metals; how atoms and molecules interact, through London dispersion forces, dipole-dipole interactions, hydrogen bonds, and covalent and ionic bonds. We have discussed how changes in energy and entropy lead to solid, liquid, and gas state changes. So far, so good, but is this really chemistry? Where are the details about chemical reactions, acids and bases, gas laws, and so forth? Not to worry—we have approached the topics in this order so that you have a strong conceptual foundation before you proceed to the nuts and bolts of chemical reactions. Without this foundation, you would just memorize whatever equations we presented, without making the connections between seemingly disparate reactions. Many of these reactions are complex and overwhelming even for the most devoted student of chemistry. The topics we have covered so far will serve as a tool kit for understanding the behavior of increasingly complex chemical systems. We will continue to reinforce these basic ideas and their application as we move on to the types of reactions that are relevant to most chemical systems.
6.1 What Is a Solution?
The first type of complex system that we will consider is a solution. You almost certainly already have some thoughts about what a solution is and you might want to take a moment to think about what these are. This will help you recognize your implicit assumptions if they “get in the way” of understanding what a solution is scientifically. The major difference between a solution and the systems we have previously discussed is that solutions have more than one chemical substance in them. This raises the question: what exactly is a solution and what does it mean to dissolve? You are probably thinking of examples like sugar or salt dissolved in water or soda. What about milk? Is it a solution? Do solutions have to be liquid or can they also include gases and solids? What is the difference between a solution and a mixture?
It turns out that we can make solutions from a wide range of materials. Although it is common to think of solutions in terms of a solid dissolved into a liquid, this is not the only type of solution. Other examples of solutions include: gas in liquid (where molecular oxygen, or O2, dissolves in water – important for fish); solid in solid (the alloy brass is a solution of copper and zinc); gas in solid (hydrogen can be dissolved in the metal palladium); and liquid in liquid (beer is a solution of ethanol and water and a few other things).
Let us take a closer look at what we mean by a solution, starting with a two-component system. Typically, one of the components is present in a smaller amount than the other. We call the major component the solvent and the minor component(s) the solute(s). The most familiar solutions are aqueous solutions, in which water is the solvent. For example, in a solution of the sugar glucose in water, glucose molecules are the solute and water molecules are the solvent. In beer, which is typically 2–4% ethanol, ethanol is the primary solute and water is the solvent. Once they are thoroughly mixed, solutions have the same composition throughout—they are homogeneous at the macroscopic scale, even though at the molecular level we still find different types of molecules or ions. This is an important point: Once mixed, they remain mixed! If you take a sample from the top of a solution, it has the same composition as a sample from elsewhere in the solution. Solutions, when viewed at the molecular level, have the solute particles evenly (and randomly) dispersed in the solute. Also, because the solute and solvent are in contact with each other, there must be some kind of molecular interaction between the two types of molecules. This is not true for simple mixtures. For example, we tend to describe air as a mixture of gases (N2, O2, H2O, etc.), rather than a solution because the gas molecules do not interact aside from the occasional collision with each other.
Molecular Formation of Solutions
Let us consider a solution of ethanol and water. Many common solutions contain these two components (usually with minor amounts of other substances as well). Ethanol and water are soluble in each other (what is known as “miscible”) in all proportions. For example, beer is typically about 3% alcohol (6% proof),[1] wine about 6% alcohol (12% proof), and liquors such as whiskey or brandy are about 50% alcohol (100% proof). How do they dissolve into each other at the molecular level, and why?
For a process to be thermodynamically favorable, the Gibbs (free) energy change (ΔG) associated with that process must be negative. However, we have learned that Gibbs energy change depends on both enthalpy (H) and entropy (S) changes in the system. It is possible to envision a wide range of situations – involving both positive and negative changes in H and S, and we have to consider the magnitudes of the enthalpy, the entropy and the temperature changes.
So what happens when we add a drop of ethanol to a volume of water? The ethanol molecules rapidly disperse and the solution becomes homogeneous. The entropy of the ethanol–water solution is higher than that of either substance on its own. In other words, there are more distinguishable arrangements of the molecules when they are mixed than when they are separate. Using simple entropic arguments we might, at least initially, extend the idea to include all solutions. Everything should be soluble in everything else, because this would to an entropy increase, right? Wrong. We know that this is not true. For example, oil is not soluble in water and neither are diamonds, although for very different reasons. So what are the factors influencing solution formation? We will see that some are entropic (involving ΔS) and some enthalpic (involving ΔH.)
Questions
Questions to Answer
- Make a list of some common solutions you might encounter in everyday life. How do you know they are solutions and not mixtures?
- Consider a solution formed from 100 g of water and 5 g sodium chloride:
- What would you expect the mass of the solution to be? Why?
- What would you expect the volume of the solution to be? Why?
- How would you test your hypotheses? What experiments would you do?
- What evidence would you collect?
6.2 Solubility: why do some things form solutions and others not?
Let us say you have a 100-mL graduated cylinder and you take 50 mL of ethanol and add it to 50 mL of water. You might be surprised to find that the volume of the resulting solution is less than 100 mL. In fact, it is about 98 mL, assuming good technique (no spilling). How can we explain this? Well, we can first reassure ourselves that matter has not been destroyed. If we weigh the solution, it weighs the same as 50 mL of water plus 50 mL of ethanol. This means that the density of the water–ethanol solution must be greater than the density of either the water or ethanol alone. At the molecular level, we can immediately deduce that the molecules are closer together in the ethanol and water mixture than they were when pure (before mixing) –try drawing a molecular level picture of this to convince yourself that this is possible. Now, if you took 50 mL of oil and 50 mL of water, you would find that they do not mix—no matter how hard you tried. They will always separate away from one another into two layers. What factors determine whether or not substances form solutions?
First, we need to be aware that solubility is not an all-or-nothing property. Even in the case of oil and water, a very small number of oil molecules are present in the water (the aqueous phase), and a small number of water molecules are present in the oil. There are a number of ways to describe solubility. The most common way is to define the number of moles of solute per liter of solution. This is called the solution’s molarity (M, mol/L). Another common way is to define the number grams of solute per mass of solution. For example: 1 mg (10-3 g) of solute dissolved in 1 kg (103 g) of solution is 1 part per million (106) solute, or 1 ppm. As you might expect, given the temperature term in the free energy equation, solubility data are always reported at a particular temperature. If no more solute can dissolve at a given temperature, the solution is said to be saturated; if more solute can dissolve, it is unsaturated.
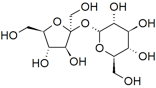
If we look at the structure of compounds that dissolve in water, we can begin to see some trends: hydrocarbons are not very soluble in water (remember from Chapter 4 that these are compounds composed only of carbon and hydrogen), whereas alcohols (hydrocarbons with an —O–H group attached) with up to 3 carbons are completely soluble. As the number of carbon atoms increases, the solubility of the compound in water decreases. For example, hexanol (CH3CH2CH2CH2CH2CH2OH), is only very slightly soluble in water (0.4 g/L). So perhaps the hydroxyl (—O–H) group is responsible for the molecule’s solubility in water. Evidence supporting this hypothesis can be found in the fact that diols (compounds with 2 —O–H groups) are more soluble than similar alcohols. For example, compared to hexanol, 1,6-hexanediol (HOCH2CH2CH2CH2CH2CH2CH2OH) is quite soluble in water. More familiar water-soluble compounds such as the sugars glucose, fructose, and sucrose (a dimer of glucose and fructose – shown in the figure) are, in fact, polyalcohols. Each of their six carbons is attached to a hydroxyl group.
| Compound | Molar Mass (g/mol) | Structure | Solubility (g/L) 20 ºC |
| Propane | 44 | CH3CH2CH3 | 0.07g/L |
| Ethanol | 46 | CH3CH2OH | Completely miscible |
| Dimethyl ether | 46 | CH3OCH3 | 328 g/L |
| Pentane | 72 | CH3CH2CH2CH2CH3 | 0.4 g/L |
| Butanol | 74 | CH3CH2CH2CH2OH | 80 g/L |
| Diethyl ether | 74 | CH3CH2OCH2CH3 | 69 g/L |
| Hexanol | 102 | CH3CH2CH2CH2CH2CH2CH2OH | 0.4 g/L |
| 1,6 Hexanediol | 226 | HOCH2H2CH2CH2CH2CH2CH2OH | 500 g/L |
| Glucose | 180 | C6H12O6 | 910g/L |
Questions
Questions to Answer
- Make a list of substances that you know dissolve in water.
- Which of these dissolve: metals, ionic compounds, molecular compounds (polar, non-polar), network solids (diamond graphite)?
- Can you make any generalizations about which things dissolve and which don’t?
- What must happen in order for something to dissolve in water?
- How would you design an experiment to determine the solubility of a solute?
- How would you determine whether or not a solution was saturated?
- Draw a molecular level picture of a solution of ethanol and water showing why the solution is more dense than the separate liquids.
- Draw a molecular level picture of an oil and water mixture.
- Draw a molecular level picture of the process of solution
- When you try mixing oil and water, which layer ends up on top? Why?
Question to Ponder
- You have a saturated solution, with some solid solute present.
- Do you think the solute particles that are in solution are the same ones over time?
- How would you determine whether they were the same?
Questions for Later
- What would you predict for the sign of ΔS upon the formation of any solution? Why?
- What would you predict for the sign of ΔH upon the formation of any solution? Why?
- What would you predict for the sign of ΔG upon the formation of any solution? Why?
6.3 Hydrogen Bonding Interactions and Solubility
How does adding hydroxyl groups increase the solubility of a hydrocarbon in water? To understand this, we must return to the two components of the free energy equation: enthalpy and entropy. For a solute to dissolve in a liquid, the solute molecules must be dispersed in that liquid. Solubility depends on how many solute molecules can be present within a volume of solution before they begin to associate preferentially with themselves rather than the solvent molecules. When the solute molecules are dispersed, whatever bonds or attractions holding the particles together in the solute are replaced by interactions between solvent and solute molecules. You might deduce that one reason diamonds are not soluble in water is that the C—C bonds holding a carbon atom within a diamond are much stronger (take more energy to break) than the possible interactions between carbon atoms and water molecules. For a diamond to dissolve in water, a chemical reaction must take place in which multiple covalent bonds are broken. Based on this idea, we can conclude that the stronger the interactions between the solute particles, the less favorable it is for the solute to dissolve in water. At the same time, the stronger the interactions between solute and solvent molecules, the greater the likelihood that solubility will increase.
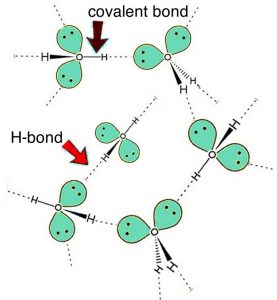
So do intermolecular interactions explain everything about solubility? Do they explain the differences between the solubility of hexane, hexanol, and hexanediol in water? Hexanediol (HO(CH2)6OH) is readily soluble, and if we consider its structure we can see that interactions between hexanediol molecules include hydrogen bonding (involving the two hydroxyl groups) and van der Waals interactions (LDFs and dipole-dipole). We can also approach this from a more abstract perspective. If we indicate the non-hydroxyl (—O–H) part of a molecule as R, then an alcohol molecule can be represented as R—O—H, and a diol can be represented as H–O—R—O–H. All alcohols have the ability to form hydrogen bonding interactions with each other as well as with water. So when an alcohol dissolves in water, the interactions between the alcohol molecules are replaced by interactions between alcohol and water molecules—an interaction similar to that between water molecules. Like water molecules, alcohols have a dipole (unequal charge distribution), with a small negative charge on the oxygen(s) and small positive charges on the hydrogen (bonded to the oxygen atoms). It makes sense that molecules with similar structures interact in similar ways. Thus, small molecular-weight alcohols can dissolve in water. But if you look again at the previous table, notice that hexanol (a 6-carbon chain with one —O–H group) is much less soluble than hexanediol (a 6-carbon chain with two —O–H groups—one at each end). As the non-polar carbon chain lengthens, the solubility typically decreases. However, if there are more —O–H groups present, there are more possible interactions with the water. This is also why common sugars, which are really polyalcohols with large numbers of —O–H groups (at least 4 or 5 per molecule), are very soluble in water. Their –O–H groups form hydrogen-bonds with water molecules to form stabilizing interactions. As the length of the hydrocarbon chain increases, the non-polar hydrocarbon part of the molecule starts to become more important and the solubility decreases. This phenomenon is responsible for the “like-dissolves-like” statements that are often found in introductory chemistry books (including this one, apparently). So, do intermolecular interactions explain everything about solubility? If only things were so simple!
Entropy and Solubility: Why Don’t Oil and Water Mix?[2]
The fact that oil and water do not mix is well known. It has even become a common metaphor for other things that do not mix (people, faiths, etc.) What is not quite so well known is, why? Oil is a generic name for a group of compounds, many of which are hydrocarbons or contain hydrocarbon-like regions. Oils are – well – oily, they are slippery and (at the risk of sounding tedious) unable to mix with water. The molecules in olive oil or corn oil typically have a long hydrocarbon chain of about 16–18 carbons. These molecules often have polar groups called esters (groups of atoms that contain C—O bonds) at one end.[3] Once you get more than six carbons in the chain, these groups do not greatly influence solubility in water, just as the single O—H groups in most alcohols do not greatly influence solubility. So, oily molecules are primarily non-polar and interact with one another as well as with other molecules (including water molecules), primarily through London dispersion forces (LDFs). When oil molecules are dispersed in water, their interactions with water molecules include both LDFs and interactions between the water dipole and an induced dipole on the oil molecules. Such dipole–induced dipole interactions are common and can be significant. If we were to estimate the enthalpy change associated with dispersing oily molecules in water, we would discover ΔH is approximately zero for many systems. This means that the energy required to separate the molecules in the solvent and solute is about equal to the energy released when the new solvent–solute interactions are formed.
Remember that the entropy change associated with simply mixing molecules is positive. So, if the enthalpy change associated with mixing oils and water is approximately zero, and the entropy of mixing is usually positive, why then do oil and water not mix? It appears that the only possibility left is that the change in entropy associated with dissolving oil molecules in water must be negative (thus making ΔG positive.) Moreover, if we disperse oil molecules throughout an aqueous solution, the mixed system spontaneously separates (unmixes). This seems to be a process that involves work. What force drives this work?
Rest assured, there is a non-mystical explanation but it requires thinking at both the molecular and the systems level. When hydrocarbon molecules are dispersed in water, the water molecules rearrange to maximize the number of H-bonds they make with one another. They form a cage-like structure around each hydrocarbon molecule. This cage of water molecules around each hydrocarbon molecule is a more ordered arrangement than that found in pure water, particularly when we count up and add together all of the individual cages! It is rather like the arrangement of water molecules in ice, although restricted to regions around the hydrocarbon molecule. This more ordered arrangement results in a decrease in entropy. The more oil molecules disperse in the water, the larger the decrease in entropy. On the other hand, when the oil molecules clump together, the area of “ordered water” is reduced; fewer water molecules are affected. Therefore, there is an increase in entropy associated with the clumping of oil molecules—a totally counterintuitive idea! This increase in entropy leads to a negative value for –TΔS, because of the negative sign. Therefore, in the absence of any other factor the system moves to minimize the interactions between oil and water molecules, which leads to the formation of separate oil and water phases. Depending on the relative densities of the substances, the oily phase can be either above or below the water phase. This entropy-driven separation of oil and water molecules is commonly referred to as the hydrophobic effect. Of course, oil molecules are not afraid (phobic) of water, and they do not repel water molecules. Recall that all molecules will attract each other via London dispersion forces (unless they have a permanent and similar electrical charge).
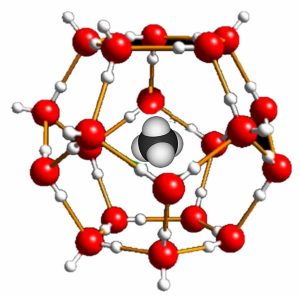
The insolubility of oil in water is controlled primarily by changes in entropy, so it is directly influenced by the temperature of the system. At low temperatures, it is possible to stabilize mixtures of water and hydrocarbons. In such mixtures, which are known as clathrates, the hydrocarbon molecules are surrounded by stable cages of water molecules (ice)(→). Recall that ice has relatively large open spaces within its crystal structure. The hydrocarbon molecules fit within these holes, making it possible to predict the maximum size of the hydrocarbon molecules that can form clathrates. For example, some oceanic bacteria generate CH4 (methane), which is then dissolved in the cold water to form methane clathrates. Scientists estimate that between two and ten times the current amount of conventional natural gas resources are present as methane clathrates.[4]
Solubility of Ionic Compounds: Salts
Polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Table salt, or sodium chloride (NaCl), the most common ionic compound, is soluble in water (360 g/L). Recall that NaCl is a salt crystal composed not of discrete NaCl molecules, but rather of an extended array of Na+ and Cl– ions bound together in three dimensions through electrostatic interactions. When NaCl dissolves in water, the electrostatic interactions within the crystal must be broken. By contrast, when molecular compounds dissolve in water, it is the intermolecular forces between separate molecules that are disrupted. One might imagine that the breaking of ionic interactions would require a very high-energy input (we have already seen that diamonds do not dissolve in water because actual covalent bonds have to be broken). That would be true if all we considered was the energy required to break the ionic interactions, as indicated by the fact that NaCl melts at 801 ºC and boils at 1413 ºC. But we know that substances like NaCl dissolve readily in water, so clearly there is something else going on. The trick is to consider the whole system when NaCl dissolves, just like we did for molecular species. We need to consider the interactions that are broken and those that are formed. These changes in interactions are reflected in the ΔH term (from ΔG = ΔH – TΔS).
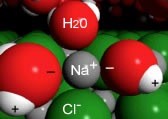
When a crystal of NaCl comes into contact with water, the water molecules interact with the Na+ and Cl– ions on the crystal’s surface, as shown in the figure. The positive ends of water molecules (the hydrogens) interact with the chloride ions, while the negative end of the water molecules (the oxygen) interacts with the sodium ions. So the ion on the surface of the solid interacts with water molecules from the solution; these water molecules form a dynamic cluster around the ion. Thermal motion (which reflects the kinetic energy of the molecules, that is the motion driven by collisions with other molecules in the system) then moves the ion and its water shell into solution.[5] The water shell is highly dynamic—molecules are entering and leaving it. The ion–dipole interaction between ions and water molecules can be very strongly stabilizing (-ΔH). The process by which solvent molecules interact with and stabilize solute molecules in solution is called solvation. When water is the solvent, the process is known as hydration.
Questions
Questions to Answer
- Draw a molecular-level picture of a solution of NaCl. Show all the kinds of particles and interactions present in the solution.
- When we calculate and measure thermodynamic quantities (such as ΔH, ΔS and ΔG), why is it important to specify the system and the surroundings?
- When a substance dissolves in water, what is the system and what are the surroundings? Why? What criteria would you use to specify the system and surroundings?
- For a solution made from NaCl and water, what interactions must be overcome as the NaCl goes into solution? What new interactions are formed in the solution?
- If the temperature goes up when the solution is formed, what can we conclude about the relative strengths of the interactions that are broken and those that are formed? What can we conclude if the temperature goes down?
- When you measure the temperature of a solution, are you measuring the system or the surroundings?
Questions to Ponder
- Why is the water shell around an ion not stable?
- What are the boundaries of a biological system?
6.4 Gibbs Energy and Solubility
Try adding NaCl to water, you can do this at the dinner table. You will see that the NaCl dissolves and the temperature of the solution goes down. Is this the case with all salts? No, it is not. If you dissolve calcium chloride (CaCl2) or magnesium chloride (MgCl2), the solution gets warmer, not colder. Dissolving CaCl2 or MgCl2 in water clearly involves some kind of energy release (recall that if the temperature increases, the average kinetic energy of the molecules in the solution also increases).
How do we explain why dissolving NaCl causes the temperature of the solution to decrease, whereas dissolving CaCl2 or MgCl2 makes the temperature increase? Because both processes (that is the dissolving of NaCl and CaCl2/MgCl2 into water) occur, they must be thermodynamically favorable. In fact, all of these compounds are highly soluble in water, the ΔG for the formation of all three solutions is negative, but the process results in different temperature changes. Let us look at the example of calcium chloride: as a crystal of CaCl2 dissolves in water, interactions between ions are broken and new interactions between water molecules and ions are formed. The table below lists the types of interactions forming in the crystal and the solvent.
Within the crystal, there are ion–ion interaction while in the solvent there are H-bonding, dipole–dipole, and LDF interactions. As the crystal dissolves, new ion–dipole interactions form between calcium ions and water molecules, as well as between chloride ions and molecules. At the same time, the majority of the interactions between water molecules are preserved.
| Interactions Present Before Solution | Interactions Present After Solution |
| ion-ion
(interactions between Ca2+ and Cl–) |
ion–dipole
interactions between Ca2+ and H2O and Cl– and H2O) |
| Interactions Between Water Molecules
H-bonding, dipole–dipole, and LDFs |
Interactions Between Water Molecules
H-bonding, dipole–dipole, and LDFs |
In order to connect our observation that the temperature increases with thermodynamic data, we have to be explicit about what we mean by the system and what we mean by the surroundings. In calcium chloride, the system is CaCl2 and the water molecules it interacts with. The surroundings are the rest of the water molecules (the solution). So when we measure the temperature change, we are actually measuring the temperature change of the surroundings (not the system). If the temperature rises, that means thermal energy is transferred from the CaCl2—H2O system to the water. Therefore, the interactions after the solution is formed are stronger and more stable than those for the solid CaCl2 and water separately. If we look up the enthalpy change for the solution of calcium chloride, it is around -80 kJ/mol: dissolving is exothermic and heat is transferred from the system to the surroundings.
So what is going on with NaCl? Solution temperatures decrease when NaCl is dissolved, so the solution (surroundings) loses energy to the ion–solvent interactions (system). Energy from the surroundings breaks up the NaCl lattice and allows ions to move into the solution. That would imply that ion–ion and H2O–H2O interactions are stronger than the ion–water interactions for the NaCl–H2O system. But why does NaCl dissolve at all? The answer is that enthalpy is not the critical factor determining whether solution happens. If we factor in the entropy change for the solution, which in this case is positive, then ΔG is negative. The dissolving of salt is an entropy-driven process!
To recap: for a solution to form, the Gibbs energy change must be negative. When calcium chloride dissolves in water, ΔH is negative and as it turns out ΔS is slightly negative (although this cannot be determined from observations). This results in a large negative ΔG and a very high solubility (595 g/L). By contrast, when sodium chloride dissolves, ΔH is positive, but ΔS is positive enough to overcome the effect of ΔH. This means that the Gibbs free energy change is also negative for this process. In fact, many solutes dissolve in water with a decrease in temperature. Ethanol—which is infinitely soluble in water—has an unfavorable enthalpy of solution. Thus, the entropy of mixing is the important factor.
Questions
Questions to Answer
- When ammonium chloride dissolves in water, the temperature of the solution drops. Predict the signs of ΔH, ΔS, and ΔG.and explain your reasoning by drawing molecular level pictures
- Calcium phosphate (Ca3(PO4)3) is insoluble in water. The ΔH for solution is about zero. Predict the signs of ΔS and ΔG and explain your reasoning by drawing molecular-level pictures.
6.5 Polarity
So far we have considered solutions that are made up of molecules that are either polar or non-polar or ionic species that have properties that are relatively easy to predict. Many substances, however, have more complex structures that incorporate polar, ionic, and non-polar groups. For example, many biomolecules cannot be classified as exclusively polar or non-polar, but are large enough to have distinct regions of differing polarity. They are termed amphipathic. Even though the structures of proteins such as RNA, DNA, and other biomolecules are complex, we can use the same principles involving entropic and enthalpic effects of interacting with water to understand the interactions between biomolecules, as well as within a given biomolecule. Biomolecules are very large compared to the molecules considered in most chemistry courses, and often one part of the molecule interacts with another part of the same molecule. The intramolecular[6] interactions of biological macromolecules, together with their interactions with water, are key factors in predicting their shapes.[7]
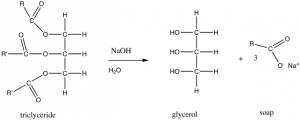
Let us begin with a relatively simple biomolecular structure. In the previous section we looked at the solubility of oils in water. Oils or fats are also known as a triglycerides. In the figure, R and R’ indicate hydrocarbon chains, which have the generic structure CH3CnH2n, shown in the figure. If you treat an oil or fat with sodium hydroxide (NaOH), the resulting chemical reaction leads to the formation of what is known as a fatty acid (in this example, oxygen atoms are maroon). A typical fatty acid has a long, non-polar hydrocarbon chain and one end that often contains both a polar and ionic group. The polar head of the molecule interacts with water with little or no increase in entropy, unlike a hydrocarbon, where the lack of H-bonding interactions with water forces a more ordered shell of water molecules around the hydrocarbon molecule, leading to a decrease in entropy. On the other hand, in water the non-polar region of the molecule creates a decrease in entropy as water molecules are organized into a type of cage around it—an unfavorable outcome in terms of ΔS, and therefore ΔG as well. So, which end of the molecule “wins”? That is do such molecules dissolve in water or not? The answer is: Both!
The polar head of the molecule interacts with water with little or no increase in entropy, unlike a hydrocarbon, where the lack of H-bonding interactions with water forces a more ordered shell of water molecules around the hydrocarbon molecule, leading to a decrease in entropy. On the other hand, in water the non-polar region of the molecule creates a decrease in entropy as water molecules are organized into a type of cage around it—an unfavorable outcome in terms of ΔS, and therefore ΔG as well. So, which end of the molecule “wins”? That is do such molecules dissolve in water or not? The answer is: Both! 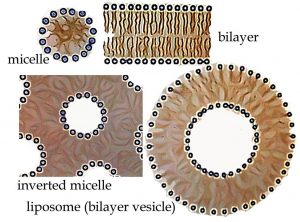 These amphipathic molecules become arranged in such a manner that their polar groups are in contact with the water, while their non-polar regions are not. (See whether you can draw out such an arrangement, remembering to include the water molecules in your drawing.)
These amphipathic molecules become arranged in such a manner that their polar groups are in contact with the water, while their non-polar regions are not. (See whether you can draw out such an arrangement, remembering to include the water molecules in your drawing.)
In fact, there are several ways to produce such an arrangement, depending in part on the amount of water in the system. A standard micelle is a spherical structure with the polar heads on the outside and the non-polar tails on the inside. It is the simplest structure that can accommodate both hydrophilic and hydrophobic groups in the same molecule. If water is limiting, it is possible to get an inverted micelle arrangement, in which polar head groups (and water) are inside and the non-polar tails point outward (as shown in the figure). Other highly organized structures can form spontaneously depending on the structure of the head group and the tail. For example, lipid molecules have multiple hydrocarbon tails and carbon ring structures called sterols. That structure creates a lipid bilayer—a polar membrane made up of two lipid molecule layers that form cellular and organellar boundaries in all organisms. It should be noted that these ordered structures are possible only because dispersing the lipid molecules in water results in a substantial decrease in the disorder of the system. In fact, many ordered structures associated with living systems, such as the structure of DNA and proteins, are the result of entropy-driven processes, yet another counterintuitive idea. This is one of the many reasons why biological systems do not violate the laws of thermodynamics and why it is scientifically plausible that life arose solely due to natural processes![8]
Questions
Questions to Answer
- If you had a compound that you suspected might form micelles:
- What structural features would you look for?
- How might you design an experiment to determine whether the compound would form micelles in water?
- What would be the experimental evidence?
- Why do you think some amphipathic molecules form spherical clusters (micelles or liposomes) whereas others form sheets (bilayers)? (Hint: consider the shape of the individual molecule itself.)
- Amphipathic molecules are often called surfactants. For example, the compounds used to disperse oil spills are surfactants. How do you think they work?
Questions to Ponder
- If membrane formation and protein folding are entropy-driven processes, does that make the origins of life seem more or less “natural” to you?
Solutions, Colloids & Emulsions
So, do micelles dissolve in water? Well, micelles are not molecules but rather supramolecular assemblies composed of many distinct molecules. A glucose solution consists of isolated glucose molecules but micelles in solution consist of larger molecular aggregates. Solutions of macromolecular solutes are called colloids. These particles can be aggregates of molecules (like micelles), atoms (nanoparticles), or larger macromolecules (proteins, nucleic acids), among others. When these particles are on the order of the wavelength of visible light, they scatter the light; smaller objects do not. This is why a salt or sugar solution is translucent, whereas a colloidal dispersion of micelles or cells is cloudy.[9] This principle also explains why soap solutions are typically cloudy—they contain particles large enough to scatter the light. When the particles in a solution maintain the structure of a solid, the end result is known as a colloid. The colloid is stable because the thermal movements of these small, solid particles are suspended. As the particles get larger, the colloid becomes unstable; the influence of gravity overcomes the effects of thermal motion and the particles settle out. Before they settle out, such unstable systems are known as suspension
But if the suspended particles are liquid, the system is known as an emulsion. For example, if we looked at a salad dressing made of oil and water under a microscope, we would see drops of oil suspended in water. Emulsions are often unstable, and over time the two liquid phases separate. This is why you have to shake up salad dressing just before using it. There are many colloids and emulsions in the world around us. Milk, for example, is an emulsion of fat globules and a colloid of protein (casein) micelles.
6.6 Temperature and Solubility
Can you also predict the effect of temperature on solubility? If you raise the temperature, does solubility of a solute increase or decrease? It would be reasonable to assume that increasing temperature increases solubility. But remember that both ΔH and ΔS have a role, and an increase in temperature increases the effect of changes in entropy. Dissolving solute into solvent is likely to increase entropy (if ΔS is positive), but this is not always the case. Consider what happens when you heat up water on the stove. Bubbles of gas are released from the liquid long before the water reaches its boiling point. At low temperatures, these bubbles contain air (primarily N2, O2) that was dissolved in the water.[10] Why? Because the solubility of most gases in water decreases as temperature rises. We can trace the reason for this back to the entropy of solution. Most gases have very small intermolecular attractions – this is the reason why they are gases after all. Gas molecules do not stick together and form solids and liquids. Therefore, they do not have very high solubility in water. As an example, the solubility of O2 in water is 8.3 mg/L (at 25 ºC and 1 atmosphere).

Most gases have a slightly favorable (negative) enthalpy of solution and a slightly unfavorable (negative) entropy of solution. The effect on enthalpy can be traced to the dipole–induced dipole attractions formed when the gas dissolves in the solution. The decrease in entropy results from the fact that the gas molecules are no longer free to roam around – their positional entropy is more constrained within the liquid phase than it is in the gas phase. When the temperature is increased the gas molecules have more kinetic energy and therefore more of them can escape from the solution, increasing their entropy as they go back to the gas phase. Thus, the solubility of O2 and other gases decreases as temperature increases. This can produce environmental problems, because less oxygen is available for organisms that live in the water. A common source of thermal pollution occurs when power plants and manufacturing facilities expel warm water into the environment.
Solutions of Solids in Solids: Alloys
Another type of solution occurs when two or more elements, typically metals, are melted and mixed together so that their atoms can intersperse, forming an alloy. Upon re-solidification, the atoms become fixed in space relative to each other and the resulting alloy has different properties than the two separate metals. Bronze was one of the first known alloys. Its major component is copper (~90%) and its minor component is tin (~10%), although other elements such as arsenic or phosphorus may also be included.
The Bronze Age was a significant leap forward in human history.[11] Before bronze, the only metals available were those that occurred naturally in their elemental form—typically silver, copper, and gold, which were not well suited to forming weapons and armor. Bronze is harder and more durable than copper because the tin atoms substitute for copper atoms in the solid lattice. Its structure has stronger metallic bonding interactions, making it harder and less deformable, with a higher melting point than copper itself. Artifacts (weapons, pots, statues, etc.) made from bronze are highly prized. Before bronze, the only metals available were those that occurred naturally in their elemental form– typically silver, copper, and gold.
Steel is another example of a solid–solid solution. It is an iron solvent with a carbon solute. The carbon atoms do not replace the iron atoms, but fit in the spaces between them; this is often called an interstitial alloy. Because there are more atoms per unit volume, steel is denser, harder, and less metallic than iron. The carbon atoms are not in the original lattice, so they affect the metallic properties more and make it harder for the atoms to move relative to each other. Steel is more rigid, less malleable, and conducts electricity and heat less effectively than iron.
Is the Formation of a Solution a Reaction?
We have not yet considered what happens during a chemical reaction: a process where the atoms present in the starting material are rearranged to produce different chemical species. You may be thinking, “Isn’t the formation of a solution a chemical reaction?” If we dissolve ethanol in water, does the mixture contain chemically different species than the two components separately? The answer is no: there are still molecules of ethanol and molecules of water. What about when an ionic substance dissolves in water? For example, sodium chloride must separate into sodium and chloride ions in order to dissolve. Is that a reaction? Certainly interactions are broken (the interactions between Na+ Cl– ions) and new interactions are made (between Na+ ions and water and Cl– ions and water), but the dissolution of a salt has not traditionally been classified as a reaction, even though it seems to fit the criteria.[12] Rather than quibble about what constitutes a reaction, let us move along the spectrum of possible changes and look at what happens when you dissolve a molecular species in water and it forms ions.
When you dissolve hydrogen chloride, HCl (a white, choking gas), in water you get an entirely new chemical substance: hydrochloric acid (or muriatic acid as it is known in hardware stores), one of the common strong acids. This reaction can be written:
HCl (g) + H2O ➞ HCl (aq)
This is a bit of shorthand because we actually begin with lots of water, but not much of it is used in the reaction. We indicate this fact by using the aq symbol for aqueous, which implies that the HCl molecules are dissolved in water (but as we will see they are now no longer molecules). It is important to recognize that hydrochloric acid, HCl (aq), has properties that are quite distinct from those of gaseous hydrogen chloride HCl (g). The processes that form hydrochloric acid are somewhat similar to those that form a solution of sodium chloride, except that in this case it is the covalent bond between H and Cl that is broken and a new covalent bond between H and O is formed at the same time.
HCl(g) + H2O ➞ H3O+ + Cl–
We call this reaction an acid–base reaction. In the next chapter, we will consider this and other reactions in (much) greater detail.
Questions
Questions to Answer
Can you convert the solubility of O2 in water into molarity (moles solute (O2) / liter solution)?
If solubility of gases depends on dipole–induced dipole interactions, what do you think the trend in solubility is for the noble gases (He, Ne, Ar, Kr, Xe)?
What else might increase the solubility of a gas (besides lowering the temperature)? (Hint: How are carbonated drinks bottled?)
Why do you think silver, copper, and gold often occur naturally as elements (rather than compounds)?
Draw an atomic-level picture of what you imagine bronze looks like and compare it to a similar picture of steel.
Use these pictures to explain the properties of bronze and steel, as compared to copper and iron.
Questions to Ponder
- Why do you think the Iron Age followed the Bronze Age? (Hint: Does iron normally occur in its elemental form? Why not?)
- How did the properties of bronze and steel influence human history?
- Percent proofing of alcoholic beverages can be traced back to the 18th century, when British sailors were partially paid in rum. To prevent it from being watered down, the rum was “proofed” by seeing if it would support the combustion of gunpowder. ↵
- Silverstein, Todd P. J. Chem. Educ. 1998 75 116 ↵
- See additional materials for structures and names of functional groups. ↵
- http://en.wikipedia.org/wiki/Methane_clathrate ↵
- ACS GenChem materials ↵
- Intramolecular means within the same molecule. Intermolecular means between or among separate molecules. ↵
- For examples, see the internet game “foldit”, which uses intramolecular interactions to predict how proteins will fold into the lowest energy shape. ↵
- Why do you use soap and shampoo? Why not use just water? The answer is, of course, that water doesn’t do a very good job of getting dirt and oil of your skin and hair because grime is just not soluble in water. Soaps and detergents are excellent examples of amphipathic molecules. They both have a polar head and a long non-polar tail, which leads to the formation of micelles. Oily molecules can then be sequestered within these micelles and washed away. ↵
- It is often possible to track the passage of a beam of light through such a solution, known as the Tyndall effect. ↵
- At the boiling point, the bubbles contain only water molecules because all the air has been expelled long before this temperature is reached. ↵
- http://en.wikipedia.org/wiki/Bronze_Age ↵
- It has been noted that one reason why chemistry is so difficult is that even experienced chemists cannot agree on the terminology and this is one such example. ↵

