1 Atoms
Melanie M. Cooper and Michael W. Klymkowsky
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generation of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling on being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied. - Nobel Laureate Richard Feynman, 1963
Most of us are quite familiar with the core principle of atomic theory—the idea that matter is composed of atoms—because we have been told that this is so since childhood. But how many of us really, and we mean really believe it, use it in our day-to-day life, understand its implications, or know the reasons why it is assumed to be true? It seems so completely and totally impossible and improbable because we do not experience atoms directly and it is easy to go through life quite successfully, at least for the vast majority of us, without having to take atoms seriously. The average person's brain is simply not wired to believe in the reality of things like atoms in a concrete and day-to-day way. Yet most scientists, and certainly most chemists, would agree that Feynman’s deceptively simple statement contains the essence of chemistry.
Atomic theory is also critical for understanding a significant number of the underlying concepts of biology and physics, not to mention geology, astronomy, ecology, and engineering. How can one sentence contain so much information? Can we really explain such a vast and diverse set of scientific observations with so little to go on? In the next two chapters we will expand on Feynman’s sentence to see just what you can do with a little imagination and thinking. At the same time, it is worth remembering that the fact that atoms are so unreal from the perspective of our day-to-day experience means that the atomic theory poses a serious barrier to understanding modern chemistry. This is a barrier that can only be dealt with if you recognize it explicitly and try to address and adjust to it. You will be rewiring your brain in order to take atoms, and their implications, seriously. We are aware that this is not an easy task. It takes effort, and much of this effort will involve self-reflection, problem-solving, and question-answering. In an important sense, you do not have to believe in atoms, but you do have to understand them.
1.1 What Do You Think You Know About Atoms?
You almost certainly have heard about atoms and it is very likely you have been taught about them. If asked you might profess to believe in their reality. You might accept that matter, in all its forms, is made up of atoms — particles that are the smallest entities that retain the identity of an element (we will discuss elements in much greater detail in the next few chapters.) It is very likely that you have been taught that atoms are made up of even smaller particles: positively charged protons, uncharged neutrons, and negatively charged electrons. You may even have heard, and perhaps even believe, that protons and neutrons can be further subdivided into quarks and gluons, while electrons are indivisible. Equally difficult to appreciate is that all atoms are organized in a very similar way, with a very tiny, but relatively heavy, positively charged nucleus surrounded by the much lighter, negatively charged electrons.
Part of the difficulty in really understanding atoms is the fact that the forces holding the atomic nucleus together, the so-called strong and weak forces, operate at such infinitesimal distances that we do not experience them directly. This is in contrast to electromagnetism and gravity, which we experience directly because they act over longer, macroscopic or visible distances. A second problem is associated with the fact that to experience the world we need to use energy; at the atomic scale the energy used to observe the system also perturbs it. This is the basis of the Heisenberg uncertainty principle, which you may have encountered or at least heard of before, and to which we will return. Finally, objects at the atomic and subatomic scales behave differently from the macroscopic objects with which we typically interact. A particle of light, a photon, an electron, a proton, or a neutron each behaves as both a particle and a wave. In terms of physics, these are neither particles nor waves; they are quantum mechanical particles. Luckily, the weirder behaviors of atomic and subatomic entities can often, but not always, be ignored in chemical and biological systems. We will touch on these topics as necessary.
Current theory holds that each atom contains a very, very small, but very dense nucleus, which contains protons and neutrons and is surrounded by electrons. These electrons are very light, relatively, but the space occupied by moving electrons accounts for the vast majority of the volume of an atom. Because the number of positively charged protons and negatively charged electrons are equal and the size of the charges are the same but opposite, atoms are electrically neutral when taken as a whole; that is, each positively-charged proton is counterbalanced by a negatively-charged electron.
Often the definition of an atom contains some language about how atoms are the smallest particle identifiable as that element. What do we mean by that? For example, can an atom have chemical properties? And how can ensembles of the same particles, that is protons, electrons, and neutrons, have different properties? This is the mystery of the atom and understanding it is the foundation of chemistry. In this first chapter, we hope to lead you to a basic understanding of atomic structure and inter-atomic interactions. Subsequent chapters will extend and deepen this understanding.
Questions
Questions to Ponder
- If you had to explain to a non-scientist why it is that scientists accept the idea that all material things are composed of atoms what evidence would you use?
- Does the ability of science to explain so much about the world influence your view about the reality of supernatural forces?
1.2 Atomic Realities and Scientific Theories
We assume that you have lots of ideas about atoms but did you ever stop to think how we came to accept this information as reasonable or what the reality of atoms implies about how the world we perceive behaves? Atoms are incredibly and unimaginably small. A gold atom with its full complement of electrons is less than a nanometer (1 x 10–9 meters) in diameter and its nucleus, which contains 79 protons and generally around 116 neutrons, has a radius of ~1.5 x 10–14 meters. While these sizes are actually unimaginable, there are a number of web-based activities that can help you come to terms with the scales of atoms.[1] There is no way you could see an atom with your eye or with a light microscope, although there are now techniques that allow us to view computer representations of individual atoms using various types of electron and force-probe microscopes. The smallest particle of matter that you can see with your naked eye contains more atoms than there are people in the world. Every cell in your body contains a huge number of atoms. Obviously, whatever we know about atoms is based on indirect evidence; we do not directly experience atoms.
The full story of how we know what we know about the existence and structure of atoms is fascinating, complex, and perhaps fortunately for you, too long to go into in detail. What we do want to do is to consider a number of key points that illustrate how our ideas of atoms arose and have changed over time. We will present the evidence that has made accepting the atomic theory unavoidable if you want to explain and manipulate chemical reactions and the behavior of matter.
Atomic theory is an example of a scientific theory that began as speculation and, through the constraints provided by careful observation, experimentation, and logical consistency, evolved over time into a detailed set of ideas that make accurate predictions and are able to explain an increasing number of diverse, and often previously unknown, phenomena. As scientists made new observations, atomic theory was adapted to accommodate and organize these observations.
A key feature of scientific ideas, as opposed to other types of ideas, is not whether they are right or wrong but whether they are logically coherent and make unambiguous, observable, and generally quantitative predictions. They tell us what to look for and predict what we will find if we look at or measure it. When we look, we may find the world acts as predicted or that something different occurs. If the world is different from what our scientific ideas suggest then we assume we are missing something important: either our ideas need altering or perhaps we are not looking at the world in the right way. As we will see, the types of observations and experimental evidence about matter have become increasingly accurate, complex, and often abstract, that is, not part of our immediate experience. Some of these observations can be quite difficult to understand, because matter behaves quite differently on the atomic and sub-atomic scale than it does in the normal, macroscopic world. It is the macroscopic world that evolutionary processes have adapted us to understand, or at least cope with, and with which we are familiar. Yet, if we are to be scientific, we have to go where the data lead us. If we obtain results that are not consistent with our intuitions and current theories, we have to revise those theories rather than ignore the data.
However, scientists tend to be conservative when it comes to revising well-established theories because new data can sometimes be misleading. This is one reason there is so much emphasis placed on reproducibility. A single report, no matter how careful it appears, can be wrong or misinterpreted and the ability of other scientists to reproduce the observation or experiment is key to its acceptance. This is why there are no miracles in science. Even so, the meaning of an observation is not always obvious or unambiguous; more often than not an observation that at first appears to be revolutionary turns out to have a simple and even boring explanation. Truly revolutionary observations are few and far between. This is one reason that the Carl Sagan (1934-1996) quote, "Extraordinary claims require extraordinary evidence" is so often quoted by scientists. In most cases where revolutionary data is reported, subsequent studies reveal that the results were due to poor experimental design, sloppiness, or some irrelevant factor. The fact that we do not all have cold fusion energy plants driving perpetual motion refrigerators in our homes is evidence that adopting a skeptical approach that waits for experimental confirmation is wise.
A common misconception about scientific theories is that they are simply ideas that someone came up with on the spur of the moment. In everyday use, the word theory may well mean an idea or even a guess, a hypothesis, or a working assumption, but in science the word theory is reserved for explanations that encompass and explain a broad range of observations. More than just an explanation, a theory must be well tested and make clear predictions relating to new observations or experiments. For example, the theory of evolution predicted that the fossil record would show evidence for animals that share many of the features of modern humans. This was a prediction made before any such fossils were found; many fossils of human-like organisms have since been and continue to be discovered. Based on these discoveries, and on comparative analyses of the structure of organisms, it is possible to propose plausible family trees, known as phylogenies, connecting different types of organisms. Modern molecular genetics methods, particularly genome (DNA) sequencing, have confirmed these predictions and produced strong experimental support for the current view that all organisms now living on Earth are part of the same family–that is, they share a common ancestor that lived billions of years ago. The theory of evolution also predicts that the older the rocks, the more different the fossilized organisms found will be from modern organisms. In rocks dated to ~410 million years ago, we find fossils of various types of fish but not the fish that exist today. We do not find evidence of humans from that period; there are, in fact, no mammals, no reptiles, no insects, and no birds.
A scientific theory is also said to be falsifiable, which doesn’t mean that it is false but rather that it may be proven false by experimentation or observation. For example, it would be difficult to reconcile the current theory of evolution with the discovery of fossil rabbits from rocks older than 300 million years. Similarly, the atomic theory would require some serious revision if someone discovered an element that did not fit into the periodic table; the laws of thermodynamics would have to be reconsidered if someone developed a successful perpetual motion machine. A theory that can be too easily adapted to any new evidence has no real scientific value.
A second foundational premise of science is that all theories are restricted to natural phenomena; that is, phenomena that can be observed and measured, either directly or indirectly. Explanations that invoke the supernatural or the totally subjective are by definition not scientific, because there is no imaginable experiment that could be done that might provide evidence one way or another for their validity. In an important sense, it does not matter whether these supernatural explanations are true or not; they remain unscientific. Imagine an instrument that could detect the presence of angels. If such an instrument could be built, angels could be studied scientifically; their numbers and movements could be tracked and their structure and behaviors analyzed; it might even be possible to predict or control their behavior. Thus, they would cease to be supernatural and would become just another part of the natural world. Given these admitted arbitrary limitations on science as a discipline and an enterprise, it is rather surprising how well science works in explaining (and enabling us to manipulate) the world around us. At the same time, science has essentially nothing to say about the meaning of the world around us, although it is often difficult not to speculate on meaning based on current scientific ideas. Given that all theories are tentative, and may be revised or abandoned, perhaps it is wise not to use scientific ideas to decide what is good or bad, in any moral sense.
As we will see, the history of atomic theory is rife with examples of one theory being found to be inadequate, at which point it must be revised, extended, and occasionally totally replaced by a newer theory that provides testable explanations for both old and new experimental evidence. This does not mean that the original theory was necessarily completely false but rather that it was unable to fully capture the observable universe or to accurately predict newer observations. Older theories are generally subsumed as newer ones emerge; in fact, the newer theory must explain everything explained by the older one and more.
Questions
Questions to Answer: Scientific Questions and Theories:
- How would you decide whether a particular question was answerable scientifically?
- How would you decide whether an answer to a question was scientific?
- What is the difference between a scientific and a non-scientific question? Provide an example of each.
Questions to Ponder
- What things have atoms in them? Air, gold, cells, heat, light?
- How do you know atoms exist?
1.3 Some History of Atomic Theory
Modern atomic theories have their roots in the thinking of ancient peoples, in particular ancient Greek philosophers who lived over 2500 years ago. At that time the cultural, economic, and intellectual climate in Ancient Greece permitted a huge surge of philosophical and scientific development, the so-called Greek miracle. While most people of that time believed that the world was ruled by a cohort of semi-rational gods a series of philosophers, beginning with Thales of Miletus (died 546 bce),[2] were intent on developing rational and non-supernatural explanations for observable phenomena such as what we are made of and where we came from. As we know now, they could not possibly have understood the underlying nature of matter because they lacked the tools to observe and experiment at the atomic scale. However, this does not mean that their ideas were simple idle speculation. The ideas they produced, although not scientific as we understand the term today, contained remarkable insights - some of which appear to be true.
This era gave birth to a new way to approach and explore natural phenomena in order to gain understanding of their complexity and diversity in terms of natural explanations. It is worth considering that such a rational approach did not necessarily have to be productive; it could be that the world is really a totally irrational, erratic, and non-mechanistic place, constantly manipulated by supernatural forces; but given that science can not address these kinds of ideas, let us just leave them to fantasy authors. The assumption that the world is ruled solely by natural forces has been remarkably productive; that is, consistent with the way the world appears to behave when we look at it dispassionately.
The ancient Greeks developed complex ideas about the nature of the universe and the matter from which it was composed, some of which were accepted for a long time. However, in response to more careful observation and experimental analysis, these ideas were eventually superseded by more evidence-based theories. In large part this involved a process by which people took old ideas seriously, and tried to explain and manipulate the world based on them. When their observations and manipulations failed to produce the expected or desired outcomes, such as turning base metals into gold, curing diseases, or evading death altogether, they were more or less forced to revise their ideas, often abandoning older ideas for newer ideas that seemed to work.
The development of atomic theories is intertwined with ideas about the fundamental nature of matter, not to mention the origin of the universe and its evolution. Most Greek philosophers thought that matter was composed of some set of basic elements, for example, the familiar earth, air, fire, and water. Some philosophers proposed the presence of a fifth element, known as quintessence or aether.[3] These clearly inadequate ideas persist today as part of astrology and the signs of the Zodiac—a poor tribute to some very serious thinkers.
The original elements, that is, earth, air, fire, and water, were thought to be composed of tiny indestructible particles, called atoms by Leucippus and Democritus (who lived around 460 bce).[4] The atoms of different elements were assumed to be of different sizes and shapes, and their shapes directly gave rise to the properties of the particular element. For example, the atoms of earth were thought to be cubic; their close packing made earth solid and difficult to move. The idea that the structure of atoms determines the observable properties of the material is one that we will return to, in a somewhat different form, time and again. Although the particulars were not correct, the basic idea turns out to be sound.
In addition to their shapes, atoms were also thought to be in constant motion, based on watching the movement of dust motes in sunlight, with nothing, or a void, between them.[5] Many centuries later Einstein's analysis of this type of motion, known as Brownian motion, provided strong experimental support for the physical reality of molecules, larger structures composed of atoms, and the relationship between molecular movement, temperature, and energy, which we will consider later on in this chapter.
All in all the combined notions of the Greek philosophers provided a self-consistent and satisfactory basis for an explanation of the behavior of matter, as far as they could tell. The trap here is one that is very easy to fall into, namely that a satisfying explanation for a phenomenon is not necessarily true. Even if it seems to be self-consistent, useful, or comforting, an explanation is not scientific unless it makes testable, quantitative predictions. For example, it was thought that different materials were made up of different proportions of the four ancient elements. Bones were made of water, earth, and fire in the proportions 1:1:2, whereas flesh was composed of these elements in a ratio of 2:1:1.[6] While these ideas are now considered strange, they contain a foreshadowing of the “law of multiple proportions”, which would come some 2300 years later and which we will deal with later in this chapter. Some philosophers even thought that the soul was composed of atoms or that atoms themselves had a form of consciousness, two ideas that seem quite foreign to (most of) us today.
Such ideas about atoms and elements provided logical and rational, that is, non-supernatural explanations for many of the properties of matter. But the Greeks were not the only ancient people to come up with explanations for the nature of matter and its behavior. In fact, it is thought that the root of the words alchemy and chemistry is the ancient Greek word Khem, the Greek name for Egypt, where alchemy and chemistry are thought to have originated.[7] Similar theories were being developed in India at about the same time, although it is the Greek ideas about atoms that were preserved and used by the people who eventually developed our modern atomic theories. With the passage of time ancient ideas about atoms and matter were kept alive by historians and chroniclers, in particular scholars in the Arab world. During the European Dark Ages and into medieval times, there were a few scattered revivals of ideas about atoms, but it was not until the Renaissance that the cultural and intellectual climate once again allowed the relatively free flowering of ideas. This included speculation on the nature of matter, atoms, and life. Experimental studies based on these ideas led to their revision and the eventual appearance of science, as we now know it. It is also worth remembering that this relative explosion of new ideas was occasionally and sometimes vigorously opposed by religious institutions, leading to torture, confinement, and executions.[8]
Questions:
Questions to Answer:
- What properties ascribed by the Greeks to atoms do we still consider to be valid?
Questions to Ponder:
- If earth had atoms that were cubic, what shape would you ascribe to the elements air, water, and fire?
Questions for Later
- If atoms are in constant motion, what do you think keeps them moving?
1.4 Identifying and Isolating Elements
The Greek notion of atoms and elements survived for many centuries and it was eventually fleshed out with the addition of a few more elements, mostly through the efforts of the alchemists. Some elements such as gold were discovered much earlier - mainly because they exist as elements rather than compounds. By the late eighteenth century, the idea of an element as a substance that cannot be broken down into more fundamental substances had begun to be accepted. In 1789 Antoine Lavoisier (1743–1794) produced a list of 33 elements. His list did not include earth, air, fire, and water, but it did contain light and heat, along with a number of modern elements including cobalt, mercury, zinc, and copper. It had already been established that oxygen and hydrogen were elements, while water was not. The stage was set for a rapid growth in our knowledge about the underlying structure of matter. We now know of 91 naturally occurring elements, and quite a number of unnatural, that is, human-made ones which are not found in nature because they unstable. These human-made elements are heavier in atomic terms than the naturally occurring elements and are typically generated by smashing atoms of natural elements into one another; they break down, or decay, rapidly into atoms of other elements. As examples of how science can remove some of the mystery from the universe: our understanding of atoms and elements means that no new natural, light elements are theoretically possible. We know of all the light elements that can possibly exist anywhere in the universe, a pretty amazing fact. Similarly, our current understanding of the theory of general relativity and the laws of thermodynamics make faster-than-light travel and perpetual motion machines impossible, although it does not stop people from speculating about them.
The first modern chemical isolation of an element is attributed to the alchemist Hennig Brand (c. 1630–c. 1710).[9] He isolated phosphorus from urine while in pursuit of the philosopher’s stone.[10] While this may seem like an odd thing to do, people have done much stranger things in pursuit of gold or cures for diseases like syphilis. Imagine his surprise when, after boiling off all the water from the urine, the residue burst into flames and gave off a gas that, when condensed, produced a solid that glowed green in the dark. It was for this reason that he named it phosphorus, from the Greek for light-bearer. Similarly, mercury was originally isolated by roasting the mineral cinnabar. Despite being quite toxic, mercury was used as a treatment for syphilis prior to the discovery of effective antibiotics.
Questions
Questions to Answer
- Given what you know, how would you explain the difference between an atom and an element?
- What differentiates one element from another?
- What is the difference between an atom and a molecule?
- What is the difference between an element and a compound?
Questions to Ponder
- What types of evidence might be used to prove you had isolated a new element?
- When can unproven/unsubstantiated assumptions be scientific?
- Under what conditions are such assumptions useful?
- Why do you think gold was recognized as an element earlier than many others?
1.5 Evidence for Atoms
It is important to note that from the time that the first ideas of atoms arose, and for thousands of years thereafter, there was not one shred of evidence for the particulate nature of matter or the physical existence of atoms. The idea of atoms was purely a product of imagination, and while there was vigorous debate about the nature of matter, this debate could not be settled scientifically until there was objective empirical evidence one way or another.
So the question arises, how did scientists in the nineteenth century eventually produce clear evidence for the existence of atoms? We have already said atoms are much too small to be seen by any direct method. So what would lead scientists to the unavoidable conclusion that matter is composed of discrete atoms? It is often the case that a huge intuitive leap must be made to explain the results of scientific observations. For example, the story about Isaac Newton (1643–1727) and the falling apple captures this truism, namely the remarkable assumption that the movement of Earth around the Sun, the trajectory of a cannon ball, and the falling of an apple to Earth are all due to a common underlying factor, the force of gravity, which acts at a distance and obeys an inverse square relationship, 1/r2 where r is the distance between two objects. This seems like a pretty weird and rather over-blown speculation; how does this “action at a distance” between two objects work? Yet, followed scientifically, it appeared to be very powerful and remarkably accurate. The point is that Newton was able to make sense of the data, something that is in no way trivial. It requires a capacity for deep, original, and complex thought. That said, it was not until Albert Einstein (1874-1955) proposed his general theory of relativity in 1915 that there was a coherent, mechanistic explanation for gravitational forces.
The first scientific theory of atomic structure was proposed by John Dalton (1766–1844), a self-taught Quaker[11] living in Manchester, England.[12] In 1805 Dalton published his atomic theory to explain the observed law of multiple, or definite, proportions, which stated briefly is “when elements combine, they do so in the ratio of small whole numbers”, we will return to this idea later on, in much greater detail.[13] Rather surprisingly, Dalton never really explained what led him to propose his atomic theory, although he certainly used it to explain existing rules about how different elements combine. Among these rules was the observation that the total matter present in a system does not change during a chemical reaction, although a reaction might lead to a change from a solid to a gas or vice versa. Dalton’s atomic theory (1805) had a number of important components:
- Elements are composed of small indivisible, indestructible particles called atoms.
- All atoms of an element are identical and have the same mass and properties.
- Atoms of a given element are different from atoms of other elements.
- Compounds are formed by combinations of atoms of two or more elements.
- Chemical reactions are due to the rearrangements of atoms, and atoms (matter) are neither created nor destroyed during a reaction.
Based on these tenets he was able to explain many of the observations that had been made, by himself and others, about how matter behaves and reacts. More modern atomic theories have made some modifications, for example to include the existence of atomic isotopes, that is, atoms with different numbers of neutrons, but the same number of protons and electrons, and the conversion of energy into matter and vice versa, but Dalton’s core ideas remain valid.
Questions
Questions to Answer
- In what ways is Dalton’s atomic theory different from the ideas of the Greek philosophers?
- Which tenets of Dalton’s theory still hold up today?
- Design an experiment to investigate whether there is a change in mass when water changes phase. What data would you collect? How would you analyze it?
Questions to Ponder
- How did Dalton conclude that there were no half-atoms?
- Which parts of Dalton's theory were unfounded speculation and which parts were based on direct observation?
1.6 The Divisible Atom
The opposite of a correct statement is a false statement. But the opposite of a profound truth may well be another profound truth. Neils Bohr (1865–1962)
Dalton’s theory of atoms as indivisible, indestructible, objects of different sizes, weights, and perhaps shapes, depending on the element, held up for almost 100 years, although there was considerable dissent about whether atoms really existed, particularly among philosophers. By 1900 the atomic theory was almost universally accepted by chemists. More evidence began to accumulate, more elements were discovered, and it even became possible to calculate the number of atoms in a particular sample. The first step, along this direction, was made by Amedeo Avogadro (1776–1856). In 1811 he proposed that, under conditions of equal temperature and pressure, equal volumes of gases contained equal numbers of particles (molecules) and that the densities of the gases, that is their weight divided by their volume, were proportional to the weight of the individual molecules. This was expanded on by the Austrian high school teacher Josef Loschmidt (1821–1895) who, in 1865, combined Avogadro's conclusion with the assumption that atoms and molecules move very much as elastic objects, think billiard balls. This enabled him to calculate the force a molecule would exert when traveling at a particular speed, something difficult to measure, and relate that to the pressure, something easily measured. In fact, this assumption enabled physicists to deduce that the temperature of a gas is related to the average kinetic energy of the molecules within it, a concept we will return to shortly.
Probing the Substructure of Atoms
The initial Greek assumption was that atoms were indivisible, essentially unchangeable from their initial creation. However, gradually evidence began to accumulate that atoms were neither indivisible nor indestructible. Evidence for the existence of particles smaller than atoms had been building up for some time, although it was not recognized as such. For example, the well-recognized phenomenon of static electricity had been known since the ancient Greeks. The name electricity comes from the Latin electricus, meaning amber-like. Rubbing amber with fur generates static electricity—the same type of spark that jumps from your finger to a doorknob or another person under dry conditions. In the late 1700s Luigi Galvani (1737–1798) discovered that animals can produce and respond to electricity, perhaps the most dramatic example being the electric eels and rays that stun their prey through electrical shocks. The discovery of bioelectricity was exploited in many novels and movies, beginning with Mary Shelly's (1797–1851) novel Frankenstein and continuing through Mel Brook's (b. 1926) comedy film, Young Frankenstein. Galvani discovered that a dead frog’s leg would twitch in response to exposure to static electricity; it appeared to come back to life, just like Frankenstein’s monster. He assumed, correctly it turns out, that electrical activity was involved in the normal movement of animals. He thought that a specific form of electricity, bioelectricity, was carried in the fluid within the muscles and was a unique product of biological systems, a type of life-specific force. We now recognize that a number of biological phenomena, such as muscle contraction and brain activity, are initiated by changes in electric fields (across membranes) and that the underlying physicochemical principles are similar to those taking place in non-biological systems.
The excitement about electricity and its possible uses prompted Alessandro Volta (1745–1827) to develop the first modern battery, now known as a voltaic pile. He alternated sheets of two different metals, such as zinc and copper, with discs soaked in salt water (brine). It produced the first steady electrical current that, when applied to frog muscles, caused them to contract. Such observations indicated that biological systems can both generate and respond to electrical currents, suggesting that bioelectricity was no different than any other form of electricity. What neither Volta nor Galvani knew was the nature of electricity. What was it, exactly, and how did it flow from place to place? What was in the spark that jumped from finger to metal doorknob, or from Benjamin Franklin's (1705–1790) kite string to his finger? What was this “electrical fluid” made of?
Progress in the understanding of the nature and behavior of electricity continued throughout the 19th century and the power of electricity was harnessed to produce dramatic changes in the way people lived and worked, powering factories, lighting houses and streets, and so on. Yet there was no deep of understanding as to the physical nature of electricity. It was known that electric charge came in two forms, positive and negative, and that these charges were conserved; that is, they could not be created or destroyed, ideas first proposed by Franklin. The electrical (charged) nature of matter was well established, but not where those charges came from or what they were.
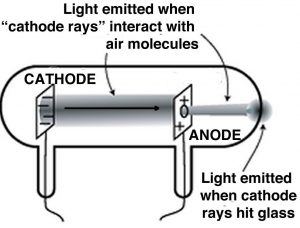
A key step to understanding electricity involved unraveling the idea of the indivisible atom and involved a series of experiments by J. J. Thompson (1856–1940), another Mancunian.[14] Although the idea of electricity was now well appreciated, Thompson and other scientists wanted to study it in a more controlled manner. They used what were, and are now, known as cathode ray tubes (CRTs). Once common in televisions, these have now been replaced by various flat screen devices. CRTs are glass tubes with wires embedded in them; these wires are connected to metal discs. The inside of the tube is coated with a chemical that glows (fluoresces) in response to electricity. They generally have ports in the walls that can be connected to a vacuum pump, so that most of the air within the tube can be removed, typically the ports are then sealed. When connected to a source of electricity, such as a voltaic pile, the fluorescent material at one end of the tube glows. In a series of experiments (1897) Thomson was able to show that:
- Rays emerged from one disc (the cathode) and moved to the other (the anode).
- “Cathode” rays were deflected by electrical fields in a direction that indicated that they were negatively charged.
- The rays could also be deflected by magnetic fields.[15]
- The rays carried the electrical charge; that is, if the ray was bent, for example by a magnetic field, the charge went with it.
- The metal that the cathode was made of did not affect the behavior of the ray; so whatever the composition of the ray, it appeared to be independent of the element that it came from.
In all of these experiments, it needs to be stressed that "positive" and "negative" are meant to indicate opposite and are assigned by convention. That means that we could decide tomorrow that positive was negative, and negative positive, and nothing would change, as long as we were consistent. From these experiments, Thompson concluded that “cathode” rays were carried by discrete charged particles, he called them corpuscles, and he assigned these particles a negative charge. But the truly stunning conclusion he reached was that these particles must come from within the atoms of the metal cathode. Because the type of metal did not affect the nature or behavior of the cathode rays, he assumed that these particles were not newly created but must pre-exist within the atoms of the cathode. Moreover, he hypothesized that identical particles must be present in all atoms, not just in the atoms of one particular metal. Do you see how he jumps from experimental results using a few metals to all elements and all atoms? Of course, we now know these particles as electrons but it is difficult to imagine what a huge impact this new theory had on scientists at the time.
Since electrons can be produced by all chemical elements, we must conclude that they enter the constitution of all atoms. We have thus taken our first step in understanding the structure of the atom. —J. J. Thomson, The Atomic Theory, 1914[16]
The discovery of the electron made the old idea of an atom as a little indestructible billiard ball-like objects obsolete and necessitated a new model. It is an example of a paradigm shift[17]—a fundamental change in scientific thinking driven by new evidence. Thompson’s first version of this new model became known as the plum pudding model.[18] His basic idea was that the atom is a ball of positively charged, but apparently amorphous, matter with electrons studded here and there, like the raisins in a pudding. Because it contained equal numbers of positive and negative charges, the overall structure was electrically neutral. Subsequent work by Thompson and Robert A. Millikan (1868–1953) established that all electrons are identical, each with the same, very small mass and negative charge. The mass of an electron is less than 1/1000th of the mass of a hydrogen atom.
Thompson's proposed plum pudding model of the atom spurred much experimental and theoretical work and led to a remarkable number of subsequent discoveries. For example, it was soon recognized that the β particles emitted by some radioactive minerals and elements, were, in fact, electrons. Other studies found that the number of electrons present in the atoms of a particular element was roughly proportional to half the element's atomic weight, although why this should be the case was unclear.
However, as more and more data began to accumulate, the plum pudding model had to be abandoned because it just could not explain what was being observed. The key experiment that led to a new model of the atom was carried out in 1908 by Ernest Rutherford (1871–1937). As you may have already guessed, he was working at the University of Manchester. In this experiment, he examined how alpha (α) particles, which he knew to be positively charged particles made of the element helium without it’s electrons, behaved when they were fired at a very thin sheet of metal, such as gold or platinum. In the experiment a narrow parallel beam of α particles was directed at a thin sheet of gold foil and the angles at which the deflected particles scattered were detected. The observed result was completely unexpected. Instead of passing straight through the thin sheet of foil, he found that a few particles were deflected, some of them at large angles. Rutherford wrote, “It is as if I had fired a cannon ball at a piece of tissue paper, and it bounced right back.” Here again, we see a particular aspect of the scientific enterprise, namely that even though only a few alpha particles bounced back, we still need to explain how this could possibly occur. We could not just say, "Only a few particles were bounced so it doesn't matter"; we have to provide a plausible scenario to explain the observation. Often it is paying attention to, and taking seriously, the unexpected result that leads to the most profound discoveries.
Based on these experimental results Rutherford reasoned that the positively charged α particles were being repelled by positive parts of the atom. Because only a very small percentage of alpha particles were deflected, only a very small region of each atom could be positively charged. That is, the positive charge in an atom could not be spread out more or less uniformly, as the plum pudding model assumed; instead it must be concentrated in a very small region. This implied that most of the atom is empty (remember the void of the ancient Greeks?) or occupied by something that poses little or no resistance to the passage of the α particles. What it left unexplained was why positively charged particles (which we now know as protons) concentrated in such a small volume, did not repel one another – the answer to which had to wait to discovery of the strong nuclear force (see below). Again we see a scientist making a huge intuitive leap from the experimental observation to a hypothesis that was consistent with that evidence and that makes specific predictions that can be confirmed or falsified by further experiment and observation. Rutherford's model, which became known as the planetary model, postulated a very, very small nucleus where all of the positive charge and nearly all of the mass of the atom was located; this nucleus was encircled by electrons. In 1920 Rutherford went on to identify the unit of positive charge and called it the proton. In 1932 James Chadwick (1891–1974)(who co-incidentally studied at the University of Manchester) identified a second component of the nucleus, the neutron. Neutrons are heavy, like protons. In fact they are slightly heavier than protons, but have no charge. The identity of the element depends on the number of protons, however the number of neutrons may be different in different atoms of the same element. For example an atom of carbon always has six protons, but it can have different numbers of neutrons. Most carbon atoms have six neutrons (C-12), but some have seven (C-13) and some have eight (C-14).
Questions
Questions for Later
- If atoms are mostly empty space, why can’t we walk through walls?
- What is radiation?
- How does an atom change when it emits an alpha particle? Or a beta particle/electron?
Questions to Ponder
- If the original discoverers of electricity had decided that electrons have a positive charge, would that have made a difference in our understanding of electricity?
- Why do you think electrons were the first sub-atomic particles to be discovered?
- How exactly did Rutherford detect alpha particles?
- Can you think of an alternative model of the atom based on Rutherford's observations?
- How would the experiment change if he had used electrons or neutrons?
1.7 Interactions Between Atoms and Molecules
At this point we have arrived at a relatively simple model of the atom. Do not to worry, we will move to more complex and realistic models in the next chapter. In this simple model the atom has a very small but heavy nucleus that contains both protons and neutrons. As we talk about biology now and again, take care not to confuse the nucleus of an atom with the nucleus of a cell; they are completely different - besides the fact that they are of very different sizes. For example, there is no barrier round the nucleus of an atom—an atomic nucleus is a clump of protons and neutrons. Surrounding the atomic nucleus are electrons, in the same number as there are protons. The atom has no net electrical charge since the number of electrons is equal to the number of protons.
Where the electrons actually are in an atom, however, is a trickier question to answer, because of quantum mechanical considerations, specifically the Heisenberg uncertainty principle, which we will return to in the next chapter. For now we are going to assume the electrons are outside the nucleus and moving. We can think of them as if they were a cloud of electron density rather than particles whizzing around (don’t worry we will provide evidence for this model soon). This simple model captures important features and enables us to begin to consider how atoms interact with one another to form molecules and how those molecules can be rearranged—real chemistry!
There are four fundamental forces that we know about at the moment: gravity, the electromagnetic force, the strong nuclear force, and the weak nuclear force. For now we can largely ignore the strong nuclear force that is involved in holding the nucleus together: .it is an attractive force between neutrons and protons and is the strongest of all known forces in the universe, ~137 times stronger than the electromagnetic force. The strong nuclear force, acts at very short ranges, ~10-15 m, or about the diameter of the nucleus. The other force involved in nuclear behavior, the weak force, plays a role in nuclear stability, specifically the stability of neutrons, but it has an even shorter range of action (10-18 m). Because the nucleus is much smaller than the atom itself we can (and will) ignore the weak and strong nuclear forces when we consider chemical interactions. The force we are probably most familiar with is gravity, which is the weakest force, more than 10-37 times weaker than the electromagnetic force, and we can ignore it from the perspective of chemistry, although it does have relevance for the biology of dinosaurs, elephants, whales, and astronauts. The electromagnetic force is responsible for almost all the phenomena that we encounter in our everyday lives. While we remain grounded on the Earth because of the gravitational interaction between our body and the Earth, the fact that we don't fall through to the center of the earth is entirely due to electromagnetic interactions. One obvious feature of the world that we experience is that it is full of solid things—things that get in each other's way. If atoms and molecules did not interact with one another, one might expect to be able to walk through walls, given that atoms are mostly empty space, but clearly this is not the case. Similarly, your own body would not hold together if your atoms, and the molecules they form, failed to interact. As we will see, all atoms and molecules attract one another—a fact that follows directly from what we know about the structure of atoms and molecules.
Questions
Questions to Ponder
- What would a modern diagram of an atom look like and what could it be used to explain?
- Why don’t the protons within a nucleus repel one another?
- Why don’t the electrons and protons attract each other and end up in the nucleus?
- How the electrons within an atom interact?
Questions for Later
- Can an atom have chemical and/or physical properties; if so, what are they?
- What are chemical and physical properties? Can you give some examples?
- What distinguishes one element from another?
Interactions Between Atoms: A Range of Effects
The attractions and repulsions between charged particles and magnets are both manifestations of the electromagnetic force. Our model of the interactions between atoms will involve only electric forces; that is, interactions between electrically charged particles, electrons and protons. In order to understand this we need to recall from physics that when charged particles come close to each other they interact. You probably recall that “like charges repel and unlike charges attract”, and that this interaction, which is known as a Coulombic interaction, depends on the sizes and signs of the charges, and is inversely proportional to the square of the distance between them (this interaction can be modeled by the equation:
[latex]F = \alpha \frac{(q_1 x q_2 )}{r^2}[/latex] (Coulomb’s Law),
where q1 and q2 are the charges on the particles and r is the distance between them. That is: there is a force of attraction (or repulsion if the two charges are of the same sign) that operates between any two charged particles. This mathematical description of the electromagnetic interaction is similar to the interaction due to gravity. That is, for a gravitational interaction there must be at least two particles (e.g. you and the Earth) and the force of the attraction depends on both masses, and is inversely proportional to the square of the distance between them:
[latex]F = \alpha \frac{(m_1 x m_2 )}{r^2}[/latex].
The difference between the two forces are: a) gravitational interactions are much weaker than electromagnetic interactions and b) gravity is solely an attractive interaction while electromagnetic interactions can be either attractive or repulsive.
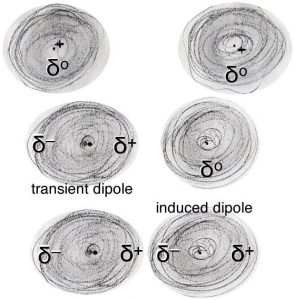
Now, let us consider how atoms interact with one another. Taken as a whole, atoms are electrically neutral, but they are composed of discrete electrically charged particles. Moreover, their electrons behave as moving objects.[19] When averaged over time the probability of finding an electron is spread uniformly around an atom, the atom is neutral. At any one instant, however, there is a non-zero probability that the electrons are more on one side of the atom than the other. This results in momentary fluctuations in the charge density around the atom and leads to a momentary charge build up; for a instant one side of the atom is slightly positive (δ+) and the other side is slightly negative (δ–). This produces what is known as an instantaneous and transient electrical dipole - that is a charge separation. As one distorted atom nears another atom it affects the second atom’s electron density distribution and leads to what is known as an “induced dipole”. So, for example, if the slightly positive end of the atom is located next to another atom, it will attract the electron(s) in the other atom. This results in an overall attraction between the atoms that varies as 1/r6 – where r is the distance between the atoms. Note that this is different than the attraction between fully charged species, the Coulombic attraction, which varies as 1/r2. What does that mean in practical terms? Well, most importantly it means that the effects of the interaction will be felt only when the two atoms are quite close to one another.
As two atoms approach, they will be increasingly attracted to one another. But this attraction has its limit - when the atoms get close enough, the interactions between the negatively charged electrons (and positively charged nuclei) of each atom increase very rapidly, which leads to an overall repulsion,which will stop the two atoms approaching so closely.
A similar effect was in also seen in Rutherford's experiment. Recall that he accelerated positively charged alpha particles toward a sheet of gold atoms. As an alpha particle approaches a gold atom's nucleus, the positively (+2) charged alpha particle and the gold atom’s positively (+79) nucleus begin to repel each other. If no other factors were involved, the repulsive force would approach infinity as the distance between the nuclei (r) approached 0. (You should be able to explain why.) But infinite forces are not something that happens in the macroscopic, atomic, or subatomic worlds, if only because the total energy in the universe is not infinite. As the distance between the alpha particle and gold nucleus approaches zero, the repulsive interaction grows strong enough to slow the incoming alpha particle and then push it away from the target particle. If the target particle is heavy compared to the incoming particle, as it was in Rutherford's experiments, the target, composed of gold atoms that weigh about 50 times as much as the alpha particle, will not move much while the incoming alpha particle will be reflected away. But, if the target and incoming particle are of similar mass, then both will be affected by the interaction and both will move. Interestingly, if the incoming particle had enough initial energy to get close enough (within ~10-15 m) to the target nucleus, then the strong nuclear force of attraction would come into play and start to stabilize the system. The result would be the fusion of the two nuclei and the creation of a different element, a process that occurs only in very high-energy systems such as the center of stars or during a stellar explosion, a supernova. We return to this idea in Chapter 3.
Questions
Questions to Answer
- How does the discovery that atoms have parts alter Dalton's atomic theory?
- What would the distribution of alpha particles, relative to the incident beam, look like if the positive nucleus took up the whole atom (sort of like the plum pudding)? What if it took up 50% of the atom?
- What does the distribution of alpha particles actually look like (recall that 1 in every 8000 particles were deflected)?
Forces and Energy: an overview.
We would like to take some time to help you think about the interactions (forces) between atoms and molecules, and how these interactions lead to energy changes. These energy changes are responsible for the formation of molecules, their reorganization through chemical reactions, and the macroscopic properties of chemical substances (i.e. everything). While you may have learned about forces and energy in your physics classes, most likely these concepts were not explicitly related to how things behave at the atomic-molecular level. We are going to begin with a discussion of the interactions and energy changes that result from the force of gravity, because these ideas are almost certainly something you are familiar with, certainly more familiar with than electromagnetic interactions – but the purpose of this section is to help you make the connections between what you already know (at the macroscopic level), and how these ideas are transferred to the molecular level, including similarities and differences. For example, Newton’s Laws of Motion describe how objects behave when they come into contact, say when a baseball comes in contact with a bat. But often objects interact with one another at a distance. After the ball is hit, its movements are determined primarily by its gravitational interactions with all other objects in the Universe, although because of the nature of the gravitational interaction, by far the most important interaction is between the ball and the Earth (see below).
A force is an interaction between objects that causes a pull (attraction) or a push (repulsion) between those objects. When such an interaction occurs, there is a change in energy of the objects. As noted above, there are four fundamental forces: gravitational, electromagnetic, the strong and the weak nuclear forces. We will have more to say about the electromagnetic force that is relevant for understanding chemical interactions, that is how atoms and molecules behave. Many of the phenomena you are familiar with are based on electromagnetic forces. For example, electromagnetic forces stop the ball from going through the bat – or you from falling down to the center of the Earth.
Now let us consider what happens when you throw a ball straight up into the air. You apply a force to the ball (through the action of your muscles), and once it leaves your hand the only force acting on the ball is gravity (we are, of course, ignoring friction due to interactions with the molecules in the air). The ball, initially at rest, starts moving upward. Over time, you observe the velocity of the ball changes, as the ball slows, stops and falls back to earth. So what forces cause these changes? The answer is the force of gravity, which is a function of the masses of the ball and the Earth, which do not change over time, and the distance (r) between the Earth and the ball, which does. This gravitational force F, can be modeled by an equation that shows it is proportional to the product of the masses of the ball (M1) and the Earth (M2) divided by the square of the distance between the objects (r).[20]
In gravitational interactions, the force decreases as the distance between the objects increases (the decrease is proportional to 1/r2), which means the further away you get from the Earth the smaller is the attractive force between you and the Earth. If you get far enough away, and you are moving away from the Earth, the interaction will not be enough to keep you attracted to the Earth and you will continue to move away forever.
Of course, why objects with mass attract each other is a subject for physics – beyond the scope of this course.[21] What we can say is that the force is mediated by a gravitational field. Any object with mass will interact with other objects with mass through this field. The field can also be said to transfer energy through space between two (or more) objects. That is, the interaction leads to an energy change in the system of interacting objects. In chemistry we are concerned with both the forces that cause interactions and the energy changes that result.
How do forces influence energy? If we take our macroscopic example of your throwing a ball upwards, we know that you transfer some energy to the ball. Of course this begs the question “what do we mean by energy?” and unfortunately we do not have an easy answer, in fact Richard Feynman once famously said “in physics we have no idea of what energy is”. Physicists might say energy is the capacity to do work, and then define work as force times distance, which does not really get us anywhere, especially in chemistry where the notion of work is often not helpful. What we can say is that any changes are accompanied by energy changes, and that we can calculate or measure these energy changes.[22]
You may be familiar with what are often referred to as “forms of energy”, such as mechanical, or elastic, or chemical, but at the most basic level all forms of energy we will be concerned with can be described either as kinetic energy, potential energy, or electromagnetic energy (e.g. light). Kinetic energy is often called the energy of motion (KE = ½ mv2, where m is the mass and v the velocity of the object), and potential energy the energy of position, or stored energy (it is calculated in various ways as we will see). Changes between kinetic and potential forms of energy involve forces. The ball that you throw straight up and then comes down has changing amounts of kinetic energy (it changes as the velocity of the ball changes) and potential energy (which changes as the distance between the Earth and the ball changes.) As the ball rises, you can observe that the velocity of the ball decreases, and therefore the KE decreases. At the same time the PE increases since the distance between the Earth and ball is increasing. On the way down the opposite is true, the ball starts moving faster – the KE increases and the PE decreases. Recall the principle of the conservation of energy; after the ball leaves your hand, no energy is added or taken away as the ball is traveling, if one form of energy increases, the other must decrease.
Another important point about energy is that it is a property of a system, rather than of an object. Although it may be tempting to consider that a ball in motion has a certain amount of kinetic energy it is important to remember the frame of reference from which you are considering the ball. Certainly the ball’s velocity is related to the KE, but that velocity depends upon where you are viewing the ball from. Usually (almost always) we consider the velocity from the point of view of an observer who is stationary, but if we changed the system we were considering, and viewed the ball while we were also moving, then the velocity of the ball would be different. This may seem quite an abstract point, but it is an important one.
Similarly it is quite tempting to say that the ball has potential energy, but in fact this is also not entirely accurate. It is more accurate – and more useful – to say that the system of the ball and the Earth has potential energy – again we are taking a systems perspective here. Unlike kinetic energy, the potential energy in a system also depends on the force that is acting on it, and that force is a function of the position of the objects that are interacting within the gravitational field. For example, a “frictionless” object traveling through a space free of fields (gravitational or otherwise) at a constant velocity has a constant kinetic energy, but no potential energy.
Potential energy (often called stored energy) or the energy of position, raises the question – where is the energy “stored”? A useful way to think about this is that for the example of the ball and the Earth, this energy is stored in the gravitational field. In this way we can accommodate the idea that the PE depends on the distance between the two interacting objects. It will also allow us to generate a more overarching concept of potential energy that will be useful in chemistry, as we extend these ideas to interactions of atoms and molecules. You might ask why then is it OK to say an object has kinetic energy (as long as you specify the frame of reference), and the difference here is that any object in motion can have energy associated with it (for example, you, an atom or a car), but potential energy must be associated with objects that are interacting via a field, be it gravitational or electromagnetic. That said, fields are everywhere – there is no place in the universe where there are no fields (although they can be balanced, leaving the net force zero). What is important here is that i) you understand that objects interact, ii) that these interactions cause a change in energy of the system, and iii) that the interacting forces depend on the distance between the interacting objects (as well as other factors, such as mass, which are constant).
The electromagnetic force: While gravitational interactions are, for all intents and purposes, irrelevant in chemistry (except to hold the beaker down on the lab bench!) they do provide a familiar example of the relationship between the kinetic and potential energies of a system that we can use to explore the electromagnetic interactions that are responsible for the behavior of atoms and molecules. There are some important similarities between gravitational and electromagnetic interactions; both act at a distance, both are mediated by fields, and both display the same relationship between force and distance. There are also important differences. In the context of chemistry, electromagnetic interactions are much stronger and while gravity is always attractive, electromagnetic interactions can be either attractive or repulsive.[23]
All electrically charged objects interact via electromagnetic forces. As we have already seen (and will to return to again) atoms and molecules are made up of charged particles (electrons and protons) and these produce unequal charge distributions that lead to the same kinds of interactions. The strength of these interactions between charged particles can be modeled using an equation, Coulomb’s Law. You will note that its form is similar to Newton’s Law of Gravitation. Instead of the masses of the two interacting objects, however, the electromagnetic force depends on the charges on the two particles (q1 and q2). The electromagnetic force typically acts over much shorter distances than gravitation, but is much stronger. It is the force that affects interactions of atoms and molecules.
As with the gravitational force as the charged particles get closer together, the interaction (whether attractive or repulsive) gets stronger. Just like gravity, the interaction between charged particles is mediated by a field, which transfers energy between interacting objects. We can identify (and calculate) the types of energy changes that are occurring as the particles interact. For example two oppositely charged particles are attracted to each other. As they approach one another, the force of attraction becomes stronger, the particles will move faster – and their kinetic energies increase. Given the fact that energy is conserved, the potential energy of the system of particles must decrease to a similar extent.[24] If, on the other hand the two charges are of the same sign, then the force between them is repulsive. So if two particles of the same charge are moving toward each other, this repulsive force will decrease their velocity (and kinetic energy), and increase their potential energy. As the distance between the particles decreases, the repulsion will eventually lead to the two particles moving away from one another.
Of course you may have noticed that there is a little problem with the equations that describe both gravitation and electromagnetic forces. If the forces change as r decreases, what happens as the distance between the interacting objects approaches zero? If we were to rely on the equations we have used so far, as r approaches 0, the force (whether repulsive or attractive) would approach infinity. Clearly something is wrong here since infinite forces are not possible (do you know why?). The ball is stopped by the surface of the Earth - it does not plummet to the center of the Earth, and charged particles do not merge into each other (or fly away at infinite speed). What is it that we are missing? Well, the problem lies in the idea that these equations are really dealing with idealized situations such as point charges or masses, rather than taking into account the fact that matter is made up of atoms, molecules and ions. When two atoms, or two molecules (or two particles made up of atoms or molecules) approach each other, they will eventually get close enough that the repulsions between like charges will become stronger than the attractive forces between unlike charges. As we will see, when two macroscopic objects appear to touch, they do not really – what stops them is the electron-electron repulsions of the atoms on the surface of the objects.[25] We will revisit all these ideas as we discuss how atoms and molecules interact at the atomic-molecular level, and how electrons behave (quantum mechanically).
Interacting Atoms: Forces, Energy Conservation and Conversion
Let us step back, collect our thoughts, and reflect on the physics of the situation. First, remember that the total matter and energy of an isolated system are conserved; that is the first law of thermodynamics. As we mentioned above, while energy and matter can, under special circumstances, be interconverted, typically they remain distinct. That means in most systems the total amount of matter is conserved and the total amount of energy is conserved, and that these are separate.
So let us consider the situation of atoms or molecules in a gas. These atoms and molecules are moving randomly in a container, colliding with one another and the container’s walls. We can think of the atoms/molecules as a population. Population thinking is useful for a number of phenomena, ranging from radioactive decay to biological evolution. For the population of atoms/molecules as a whole, there is an average speed and this average speed is a function of the temperature of the system.[26] If we were to look closely at the population of molecules, however, we would find that some molecules are moving very fast and some are moving very slowly; there is a distribution of speeds and velocities (speed + direction).
As two atoms/molecules approach each other they will feel the force of attraction caused by the electron density distortions, these are known as London dispersion forces, which we will abbreviate as LDFs. The effects of these LDFs depend on the strength of the interaction (that is the magnitude of the charges and the distance between them) and on the kinetic energies of the atoms and molecules. LDF are one of a number of intermolecular forces (IMFs), which we will consider later. LDFs are the basis of van der Waals interactions in biological systems.
To simplify things we are going to imagine a very simple system: assume for the moment that there are just two isolated atoms, atom1 and atom2. The atoms are at rest with respect to one another, but close enough that the LDF-based attractive interactions between them are significant. For this to occur they have to be quite close, since such attractive interactions decreases rapidly, as 1/r6 where r is the distance between the two atoms. At this point, the system, which we will define as the two atoms, has a certain amount of energy. The exact amount does not matter, but as long as these two atoms remain isolated, and do not interact with anything else, the energy will remain constant.
So what does all this have to do with atoms approaching one another? We can use the same kinds of reasoning to understand the changes in energy that occur as the atoms approach each other. Initially, the system will have a certain amount of energy (kinetic + potential). If the atoms are close enough to feel the effects of the attractive LDFs, they begin to move toward each other, think of a ball falling towards the Earth, and some of the potential energy associated with the atoms’ initial state is converted into kinetic energy (EK = ½mv2).
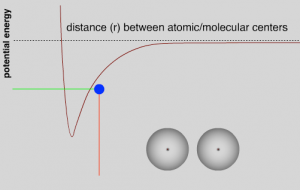
As they approach each other the LDFs grow stronger, the atoms are more strongly attracted to each other; the system’s potential energy decreases and is converted into kinetic energy, the atoms move faster.[27] The total energy remains the same as long as there are no other atoms around. This continues until the atoms get close enough that repulsive interactions between the electrons become stronger and as they approach even more closely the repulsive interactions between the positively charged nuclei also come into play, causing the potential energy in the system to rise. As the atoms begin to slow down their kinetic energy is converted back into potential energy. They will eventually stop and then be repelled from one another. At this point potential energy will be converted back into kinetic energy. As they move away, however, repulsion will be replaced by attraction and they will slow; their kinetic energy will be converted back into potential energy.[28] With no other factors acting within the system, the two atoms will oscillate forever. In the graph showing potential energy versus the distance between the atoms, we see that the potential energy of the system reaches a minimum at some distance. Closer than that and the repulsive electromagnetic forces come into play, further away and the attractive electromagnetic forces (LDF’s) are dominant. The distance between the two atoms is a function of the relative strengths of the attractive and repulsive interactions. However, even at the minimum, there is some potential energy in the system, stored in the electromagnetic field between the two atoms. At temperatures above absolute zero (0 K), the pair of atoms will also have kinetic energy – as they oscillate back and forth.
Here we have a core principle that we will return to time and again: a stabilizing interaction always lowers the potential energy of the system, and conversely a destabilizing interaction always raises the potential energy of the system. In an isolated system with only two atoms, this oscillation would continue forever because there is no way to change the energy of the system. This situation doesn’t occur in real life because two-atom systems do not occur. For example, even in a gas, where the atoms are far apart, there are typically large numbers of atoms that have a range of speeds and kinetic energies present in the system. These atoms frequently collide and transfer energy between one another. Therefore, when two atoms collide and start to oscillate, some energy may be transferred to other particles by collisions. If this happens, a stable interaction can form between the two particles; they will “stick” together. If more particles approach, they can also become attracted, and if their extra energy is transferred by collisions, the particles can form a bigger and bigger clump.
As we discussed earlier, LDFs arise due to the fluctuations of electron density around nuclei and are a feature common to all atoms; all atoms/molecules attract one another in this manner. The distance between atoms/molecules where this attraction is greatest is known as the van der Waals radius of the atom/molecule. If atoms/molecules move closer to one another than their van der Waals radii they repel one another. The van der Waals radius of an atom is characteristic for each type of atom/element. As mentioned earlier, it is only under conditions of extreme temperature and pressure that the nuclei of two atoms can fuse together to form a new type of atom; such a nuclear/atomic fusion event results in the interconversion of matter into energy.[29]
Questions
Questions to Answer
- What is potential energy? Can you provide an example?
- What is kinetic energy? Can you provide an example?
- At the atomic level, what do you think potential energy is?
- At the atomic level, what do you think kinetic energy is?
- Why does raising the temperature affect the speed of a gas molecule?
Questions to Ponder
- What is energy (have your ideas changed from before)?
Questions for Later:
- When we talk about potential energy of a system, what does system mean?
- Helium liquefies at around 4K. What makes the helium atoms stick together? (Why don’t they turn into a gas?)
- Consider two atoms separated by 1 spatial unit versus 4 spatial units. How much weaker is the interaction between the more distant atoms? How does that compared to the behavior of simple charges (rather than atoms)?
1.8 Interactions Between Helium Atoms and Hydrogen Molecules
Now let’s take a look at a couple of real systems. We begin by considering interactions between the simplest atoms, hydrogen (H) and helium (He), and the simplest molecule, molecular hydrogen (H2). A typical hydrogen atom consist of one proton and one electron, although some contain one or two neutrons and form “isotopes” known as deuterium and tritium, respectively. A hydrogen molecule is a completely different chemical entity: it contains two hydrogen atoms, but its properties and behavior are quite different. Helium atoms have 2 protons and 2 neutrons in their nuclei, and 2 electrons in their electron clouds. We will consider more complicated atoms and molecules after we discuss atomic structure in greater detail in the next chapter. One advantage of focusing on molecular hydrogen and helium is that it also allows us to introduce, compare, and briefly consider both van der Waals interactions (due to IMFs) and covalent bonds; we will do much more considering later on.
When two atoms of helium approach each other LDFs come into play and a attractive interaction develops. In the case of He the drop in potential energy due to the interaction is quite small, that is, the stabilization due to the interaction, and it does not take much energy to knock the two atoms apart. This energy is delivered by collisions with other He atoms. In fact at atmospheric pressures, Helium is never a solid and liquid He boils at ~4 K (−268.93ºC), only a few degrees above absolute zero or 0 K (−273.15 ºC).[30] This means that at all temperatures above ~4 K there is enough kinetic energy in the atoms of the system to disrupt the interactions between He atoms. The weakness of these interactions means that at higher temperatures, above 4 K, helium atoms do not “stick together”. Helium is a gas at temperatures above 4 K.
Now let us contrast the behavior of helium with that of hydrogen (H). As two hydrogen atoms approach one another they form a much more stable interaction, about 1000 times stronger than the He–He London dispersion forces. In an H–H interaction the atoms are held together by the attraction of each nucleus for both electrons. The attractive force is much stronger and as the atoms get closer this leads to a larger drop in potential energy and a minimum for the two interacting hydrogen atoms that is much deeper than that for He–He. Because of its radically different stability the H–H system gets a new name; it is known as molecular hydrogen or H2 and the interaction between the H atoms is known as a covalent bond. In order to separate a hydrogen molecule back into two hydrogen atoms, that is, to break the covalent bond, we have to supply energy.[31] This energy can take several forms: for example, energy delivered by molecular collisions with surrounding molecules or by the absorption of light both lead to the breaking of the bond.
Each H can form only a single covalent bond, leading to the formation of H–H molecules, which are often also written as H2 molecules. These H–H molecules are themselves attracted to one another through LDFs. We can compare energy associated with the H–H covalent bond and the H2 - H2 IMF. To break a H–H covalent bond one needs to heat the system to approximately 5000 K. On the other hand to break the intermolecular forces between separate H2 molecules, the system temperature only needs to rise to ~20 K; above this temperature H2 is a gas. At this temperature the IMFs between individual H2 molecules are not strong enough to resist the kinetic energy of colliding molecules. Now you may ask yourself, why does H2 boil at a higher temperature than He? Good question! It turns out that the strengths of LDFs depend on several factors including shape of the molecule, surface area, and number of electrons. For example the greater the surface areas shared between interacting atoms or molecules the greater the LDFs experienced and the stronger the resulting interaction. Another factor is the ability of the electron cloud to become charged, a property known as polarizability. You can think of polarizability as the floppiness of the electron cloud. As a rough guide, the further away from the nucleus the electrons are, the more polarizable (floppy) the electron cloud becomes. We will return to this and related topics later on. As we will see, larger molecules with more complex geometries, such as biological macromolecules (proteins and nucleic acids), can interact through more surface area and polarizable regions, leading to correspondingly stronger interactions.
At this point, you are probably (or should be) asking yourself some serious questions, such as, why don’t helium atoms form covalent bonds with one another? Why does a hydrogen atom form only one covalent bond? What happens when other kinds of atoms interact? To understand the answers to these questions, we need to consider how the structure of atoms differs between the different elements, which is the subject of the next chapter.
Questions
Questions to Answer
- Can you draw a picture (with about 20 helium atoms, represented as circles) of what solid helium would look like if you could see it?
- How would that differ from representations of liquid helium or gaseous helium?
- Now make a similar drawing of H2. Does this help explain the higher melting point of H2?
Question to Ponder
- How do the properties of solids, liquids, and gases differ?
- Scale of the universe: http://htwins.net/scale2/ ↵
- http://www.iep.utm.edu/t/thales.htm ↵
- Of course if you know your movies, you know that the “Fifth Element” is love. ↵
- http://plato.stanford.edu/entries/democritus/ ↵
- First description of Brownian motion - Epicurus ↵
- A History of Greek Philosophy by William Keith Chambers Guthrie. p. 212. ↵
- http://www.chm.bris.ac.uk/webprojects2002/crabb/history.html and http://en.wikipedia.org/wiki/Chemistry_(etymology) ↵
- An important event was the rediscovery by Poggio of Lucretius’s “On the Nature of Things,” a poem centered on the atomic nature of the universe (see The Swerve by Stephen Greenblatt). One reason Giordano Bruno was burnt at the stake was the fact that he took these ideas seriously. ↵
- http://elements.vanderkrogt.net/elem/p.html ↵
- The Philosopher’s stone was thought to be able to turn base (common) metals into gold, and perhaps even be the key to everlasting life. It was the ultimate goal of the alchemists. Interestingly the first Harry Potter book was titled Harry Potter and the Philosopher’s Stone in England but was re-titled in America, because the publishers thought that American children would not be interested in a book with this title, perhaps due a failure to appreciate the importance of philosophy. ↵
- Religious dissenters, that is, non-Anglicans, were not allowed access to English universities at that time. ↵
- An extraordinary number of discoveries related to the structure of the atom were made by scientists in or from Manchester. There must be something in the air there. It is, of course, completely fortuitous that one of the authors was also born and bred in Manchester! ↵
- https://en.wikipedia.org/wiki/Law_of_multiple_proportions ↵
- That is, a person from Manchester, England. ↵
- This works because the electrons are spinning. ↵
- http://www.aip.org/history/electron/jjsound.htm ↵
- A term made popular (although often misunderstood) by T. S. Kuhn, The Structure of Scientific Revolutions, 1st. ed., Chicago: Univ. of Chicago Pr., 1962 ↵
- This can be a little confusing to those not familiar with plum pudding – a “delicious” English delicacy composed of dried fruit (raisins) in a spongy base, usually prepared by boiling for several days and often served with rum sauce. ↵
- Yes we did tell you to think of electrons as a cloud - because this is a helpful model - but electrons are both particles and “clouds” as we will discuss later, in fact in some instances they appear to be quite close to perfect spheres in shape, In fact “The experiment, which spanned more than a decade, suggests that the electron differs from being perfectly round by less than 0.000000000000000000000000001 cm. This means that if the electron was magnified to the size of the solar system, it would still appear spherical to within the width of a human hair. (Hudson et al "Improved measurement of the shape of the electron" DOI: 10.1038/nature10104). ↵
- See http://www.youtube.com/watch?v=p_o4aY7xkXg for an excellent explanation of this phenomenon. ↵
- That said, we recommend the discription given in Einstein and Infeld’s Evolution of Physics: https://archive.org/details/evolutionofphysi033254mbp ↵
- The trouble with chemical energy: why understanding bond energies requires an interdisciplinary systems approach. CBE Life Sci. Education,12:306-12. ↵
- Magnetic, like electrical force can also be attractive or repulsive. Most of us have played with magnets and felt the force of attraction between a north and south pole of a set of magnets, which gets stronger as the magnets get closer together, and the repulsion between two north poles which also gets stronger as the magnets get closer together. ↵
- A point we have not considered is why the atoms or molecules stop moving toward each other, which will return to shortly. ↵
- http://www.youtube.com/watch?v=BksyMWSygnc ↵
- Remember speed is a directionless value, while velocity involves both speed and direction. ↵
- Imagine, as an analogy that the two atoms are balls rolling down opposite sides of a hill towards a valley, their potential energy falls as they move down - but their kinetic energy rises and they speed up. ↵
- To continue our analogy as the balls get to the bottom of the hill, they collide and bounce back - rolling back up the hill, until once again the force of gravity takes over and they start to roll back down. In an ideal (unreal) situation with no friction, this situation would simply continue, until some other factor is introduced. ↵
- It is these factors that made the report of cold fusion so strange and so exciting to physicists. The temperatures and pressures required for fusion are so high that they are extremely difficult to achieve under controlled conditions. The failure to reproduce the original cold fusion report reinforces our understanding of how atoms interact. That scientists around the world attempted to reproduce the original observation (and failed), illustrates the open-mindedness of the scientific community. The fact that badly controlled and irreproducible observations were published, illustrates how scientific effort and resources (that is, research funds) can be wasted by inadequate pre-publication review. But science, like all human activities, is imperfect. The price for open-mindedness may be be wasted time and effort, yet it remains critical to scientific process and progress. At the same time, once the replication efforts failed, it became a waste of time (or a delusional obsession) to pursue cold fusion. ↵
- According to Robert Parson, “At 1 atmosphere pressure, Helium does not melt at ANY temperature - it stays liquid down to absolute zero. (If you want to be picky, it is a liquid down to the lowest temperatures that anyone has ever achieved, which are orders of magnitude less than 1 K (http://en.wikipedia.org/wiki/Dilution_refrigerator), and our best theories predict that it will remain a liquid no matter how low the temperature.) To get solid helium you have to increase the pressure to 25 atmospheres or above. This is one of the most dramatic consequences of zero-point energy: the intermolecular forces in He are so weak that it melts under its own zero point energy. (This leads to the peculiar consequence that Helium at zero Kelvin is a liquid with zero entropy.) ↵
- In fact this is known as the bond energy – the energy required to break the bond – which in the case of H2 is 432 kJ/mol. ↵

