Part 2: Safety Information
3 Using a RAMP Approach to Learn about Hazard and Risk
R: Recognize hazards
A: Assess the risks of hazards
M: Minimize the risks of hazards
P: Prepare for emergencies
This RAMP framework for laboratory safety is derived from the work of Hill and Finster[1] and supported by the American Chemical Society (ACS), a US-based professional network of chemists. For this course, as a part of the Planning and Carrying Out Investigations (aka Planning Documents) portion of each session within a course project, your team will engage directly with these principles in your planning as well as uphold them during your team’s investigations.
R: Recognize Hazards
The first principle is to recognize the presence of hazards and the risks that they pose. This section will differentiate hazard and risk, identify the key sources of hazard information, and characterize different classes of hazards.
Defining a Hazard and a Risk
A hazard is any source of potential damage or harm to a person’s health.[2] It is an intrinsic property of the material (and/or process). These hazards will be classified into broad groups below. It is important to realize that hazards are not limited to chemicals (i.e., non-chemical equipment may potentially pose hazards), such as the potential damage from broken glass or the damage over time from carrying out repetitive motions, such as lifting or small movements as pipetting. We can never eliminate a hazard, but we can manage the risks that hazards pose.
A risk is the probability of harm or damage from a hazard.[3] It is the combination of the likelihood of an event (the exposure) and the severity of the hazard or damage that event poses.[4]

As we noted above, the hazard is an intrinsic property and cannot be eliminated, but we can minimize the likelihood of the event in which the hazard can cause damage, thereby minimizing the risk. Therefore, the emphasis is on probability because the risk can be managed through careful planning, preparation, and Personal Protective Equipment (PPE).
Where to Find Information About Hazards?
(Materials) Safety Data Sheets (M)SDS
Safety Data Sheets (SDS), formerly called Materials Safety Data Sheets (MSDS), provide information including properties, handling, and hazards of materials. As you plan your investigations, be sure to thoroughly read the SDS for each new chemical you use. In the appropriate space on the Planning Documents, you should list each chemical or material necessary for your plan, and explicitly recognize the hazards these materials pose on the appropriate line.
There are numerous sources of (M)SDS on the internet. Using a search engine, input the chemical name plus the term MSDS or SDS (e.g., “MSDS Sodium Chloride” or “SDS Isopropyl Alcohol”). If you always want to use SDS from the same source for consistency of SDS layout, you may want to search for the SDS from a particular chemical manufacturer, such as Flinn Scientific or Sigma-Aldrich. While every manufacturer may have a slightly different arrangement to their (Materials) Safety Data Sheets, or (M)SDS, each should contain all of the same sections of information. The Hazard Communication Standard Safety Data Sheets link should help you to navigate any SDS: OSHA Brief Hazard Communication Standard: Safety Data Sheets (PDF).
As you peruse the SDS for the chemicals and materials that your team decides that is necessary for your investigation, use these sheets to collect the relevant hazard information (explicated below) as well as information about proper PPE and waste disposal. You will be required to note any PPE necessary, even if it’s “appropriate goggles and nitrile gloves”. Your instructor will review your chemical safety plan and make recommendations.
Note: To foster a culture of safety in the laboratory, safety information about each project will be outlined in the following format (yellow background, red border) in the scenario. This information will cover the known, required chemical and physical materials that the project calls for. Your team—with the support of your instructor—will add to this information during your Planning Documents to account for the specifics of your team’s unique investigation and choices.

Classifying Hazards
Of the hazard information contained on an SDS, there are two broad systems of communicating hazard information you should be aware of: the NFPA diamond and the GHS labels. These systems should help you enumerate the hazards posed by the materials in your Planning Documents. In the green chemistry case studies associated with this laboratory course, the following information is helpful for evaluating sustainability solutions using green chemistry tools and metrics (described in Twelve Principles of Green Chemistry).

National Fire Protection Association (NFPA) Diamond
This ubiquitous NFPA[5] diamond summarizes major classifications of hazards in a visual manner. The individual diamonds will have numbers of symbols in them to communicate at a glance which hazards are present and the severity of the risks that they pose. The health (blue), fire (red), and reactivity (yellow) diamonds communicate hazards on a scale of 0 (least hazardous) to 4 (most hazardous) within their respective categories, and the white diamond uses abbreviations to communicate Special hazards.
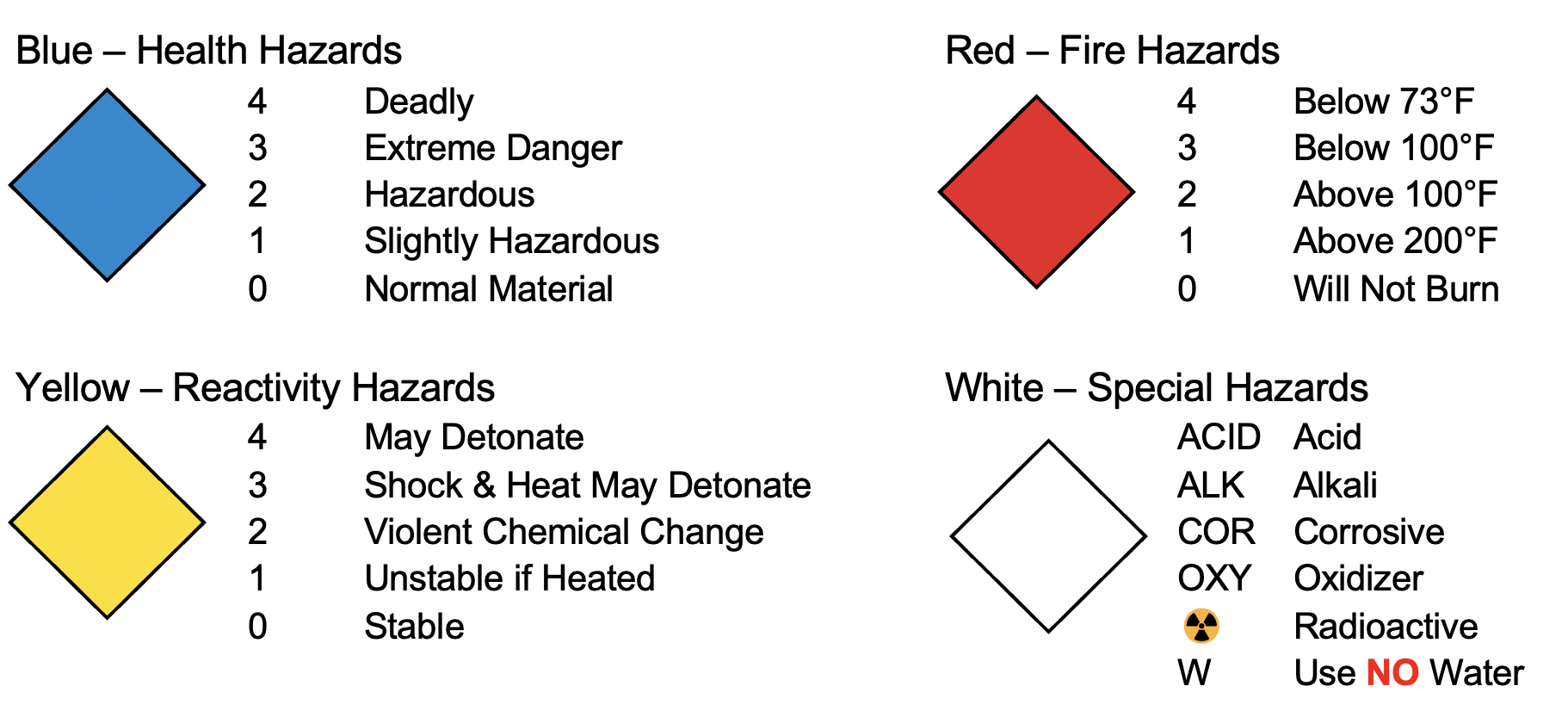

Globally Harmonized System (GHS)
The GHS labels are an internationally adopted system[6] of graphic labels intended to provide hazard information at a glance. The base template (shown at the right) of a white diamond with a thick red border will be filled with a specific graphic to indicate the class of hazard that is posed by the material.
Note that the numbering system for GHS is opposite of NFPA: a 1 is more hazardous than a 4.
The labels could be grouped into broader categories of hazards, although there are more sophisticated ways of classifying these hazards. For this course, one major category of hazards will be those posed by the chemicals or physical materials used to carry out reactions, workups, and overarching investigations. The chemical hazards are inherent properties, and include toxicity, flammability, corrosivity, and reactivity. The first group of GHS symbols below emphasis categories of hazards that fall under the broad umbrella of hazards posed by reactivity or conditions like the high pressure of compressed gas canisters. These properties have implications for shipping, storage, use, and waste generation.
Chemical Hazards
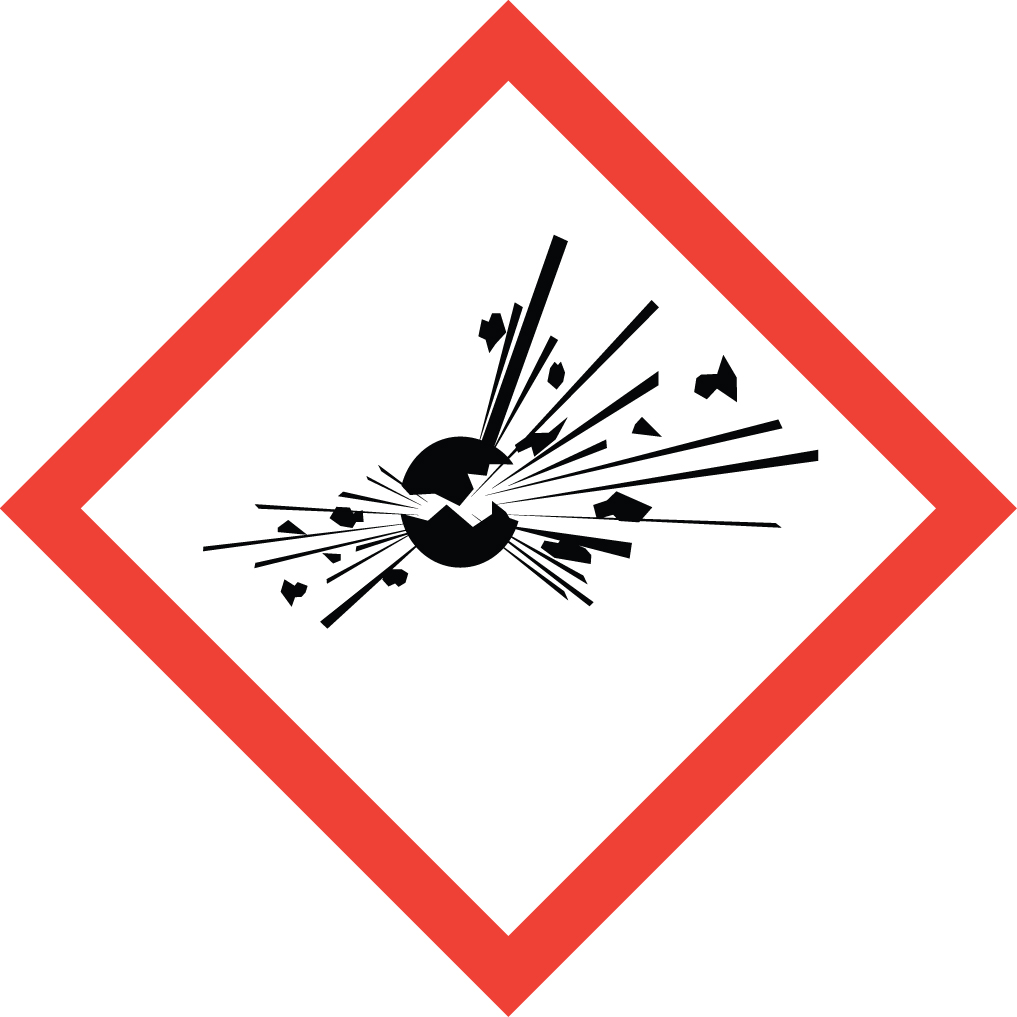
Explosive:
reacts spontaneously, exothermically & uncontrollably
- Explosives
- Self-reactive substances
- Organic peroxides
Flammable:
ignites and burns if exposed to source of ignition
- Flammable gases, aerosols, liquids, and solids
- Pyrophoric liquids or solids
- Self-heating substances
- Self-reactive substances
- Substances that emit a flammable gas upon contact with water
- Organic peroxides
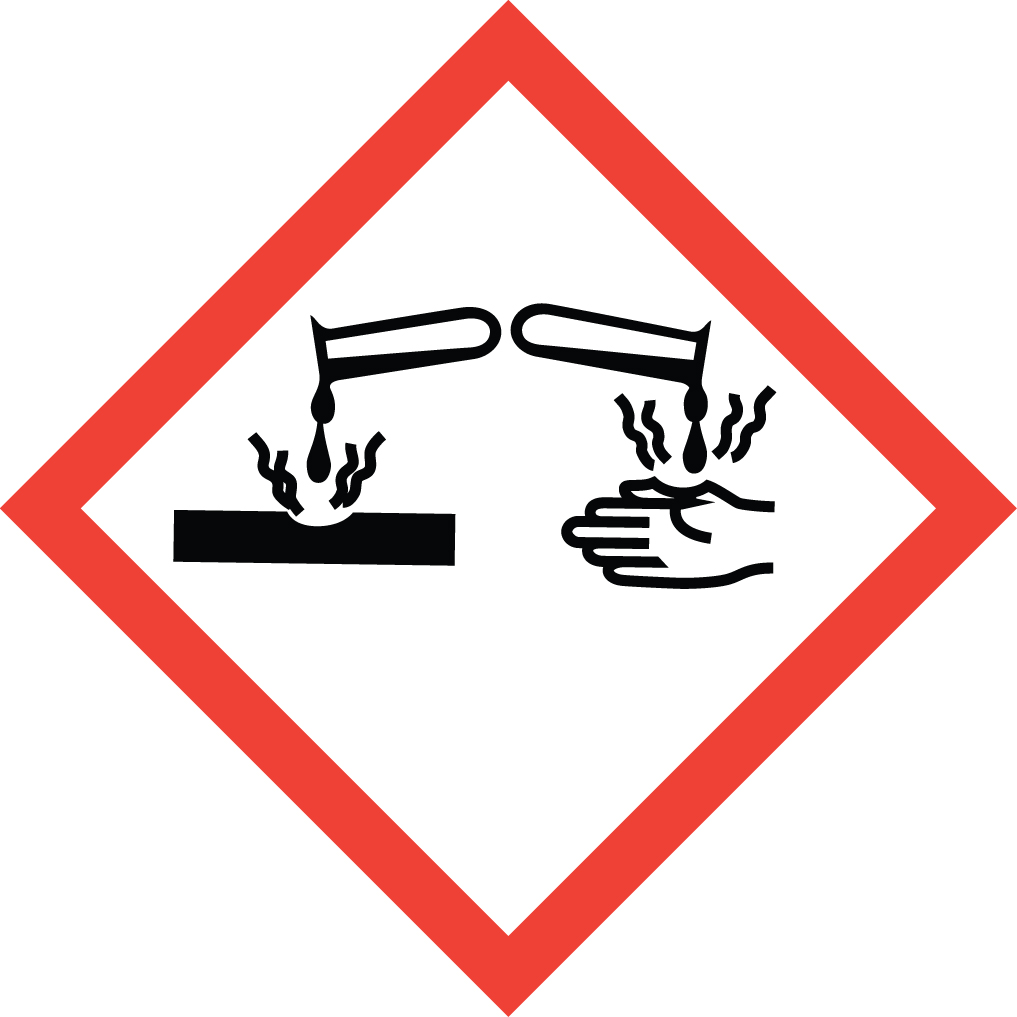
Corrosive:
reacts and damages materials and body tissues
- Skin corrosion/burns
- Eye damage
- Corrosive to metals
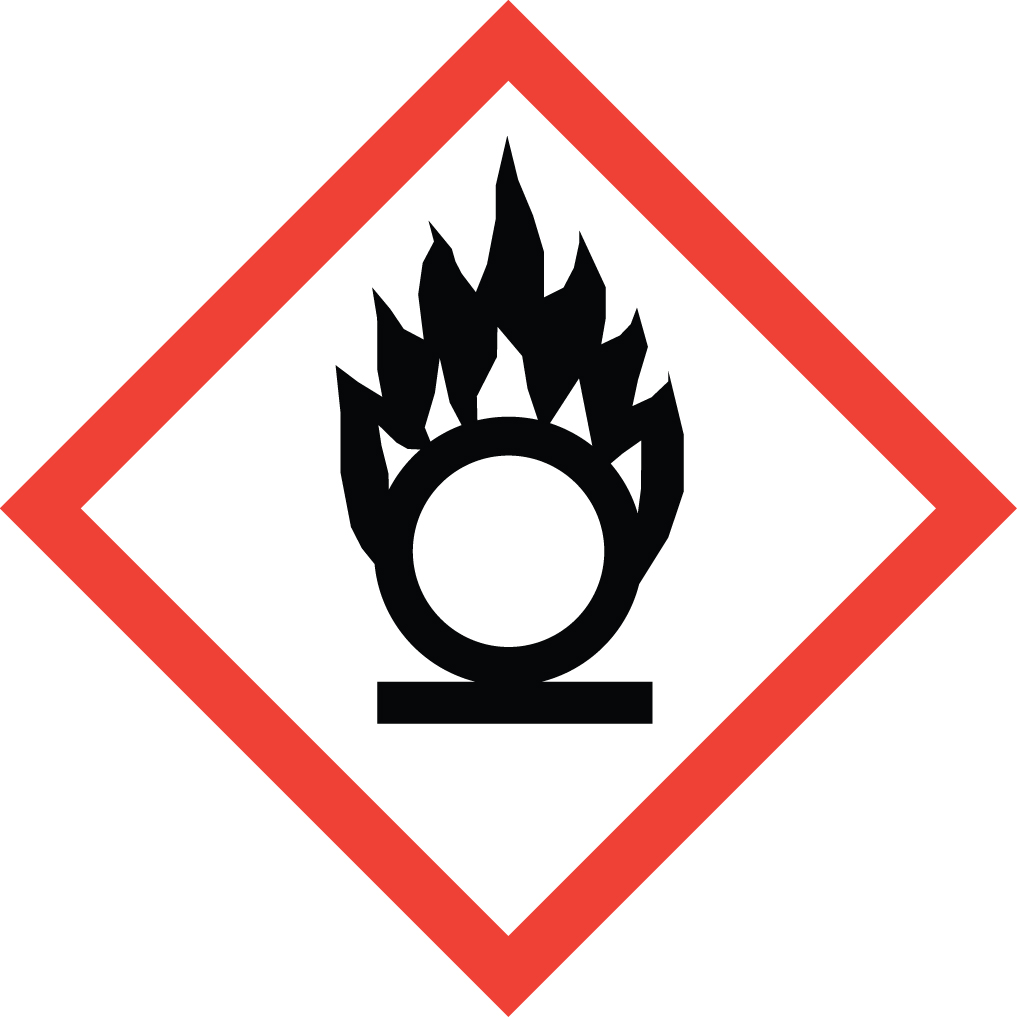
Oxidizer:
reacts readily with organic compounds or reducing agents without energy input
- Oxidizing gases, liquids, and solids

Compressed Gas:
canisters of highly pressurized gas
- Gases under pressure
Health Hazards
Health hazards include the biological and chemical materials that pose a hazard to human or environmental health. These hazards are an issue all along the life cycle of a chemical, but for our purposes we are most concerned about safe use and proper disposal. As you compile the SDS for the necessary chemicals for planning your investigations, pay special attention to the following GHS symbols. Notice how corrosion is also on this list, as it poses a human health hazard.

Toxic:
poisonous or capable of damage or death if ingested
- Acutely toxic substances may be fatal or toxic if inhaled, ingested, or absorbed through the skin

Irritant:
causes reversible inflammation or irritation to body tissue
- Irritant (skin and eye)
- Skin sensitizer
- Acute toxins
- Narcotic effects
- Respiratory tract irritants
- Hazardous to the ozone layer (non-mandatory)

Corrosive:
able to burn or corrode organic tissue by chemical action
- Skin corrosion/burns
- Eye damage
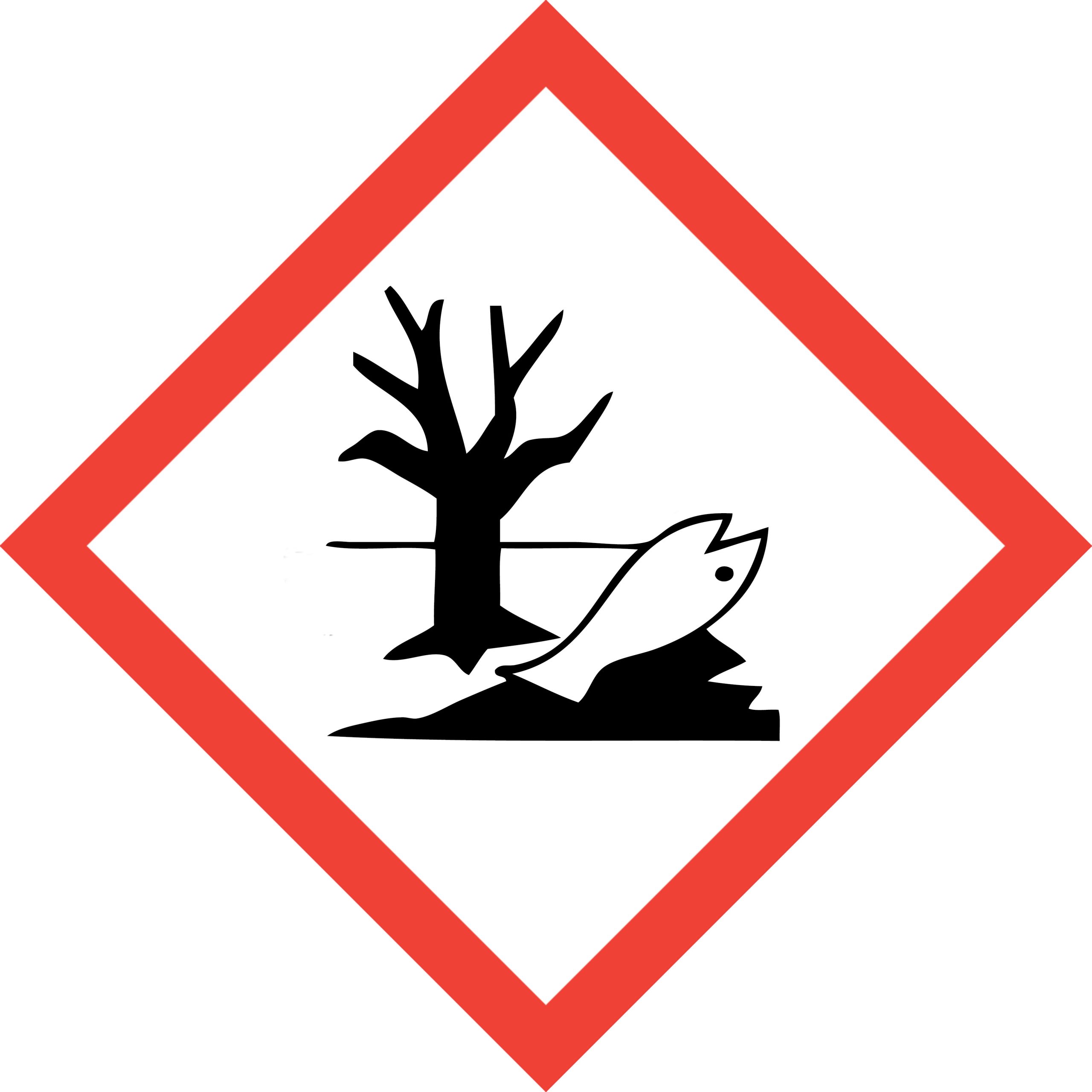
Environmental Hazard:
poses a known hazard to the environment
- Acute aquatic toxins
- Chronic aquatic toxins

Health Hazard:
poses a known hazard to human health
- Respiratory sensitizers
- Carcinogens
- Mutagens
- Reproductive toxins
- Target organ toxins, single or repeated exposure
- Aspiration toxins
Note: All GHS pictograms Copyright © United Nations, 2023. Reprinted with the permission of the United Nations.
Waste Disposal
After you have finished your experiments, dispose of waste and excess as instructed. Do not put anything down the sink unless specifically told to do so by your instructor. It is your responsibility to indicate on the planning document the appropriate waste disposal methods for each chemical; this information is available on the Safety Data Sheet (SDS). The stockroom will provide the appropriate waste containers for the relevant categories of hazards listed above.
A: Assess the Risks of Hazards
While some of these terms may be (incorrectly) used interchangeably, each class of hazards could co-occur. Some toxins—proteins from plants, animals, or bacteria that are toxicants or toxic substances—are also listed as human health hazards because of their carcinogenicity, mutagenicity, or targeting of specific organs. However, some toxins are only acute and are classified as irritants. Other toxic substances pose a chronic hazard; this distinction between “acute” and “chronic” will be explore further in the next section.
Toxicology and Green & Sustainable Chemistry
As we explore sustainability issues within the course and apply our chemistry knowledge to our everyday lives, it’s important to recognize that the environmental and human health hazards have a dose-dependent relationship to the deleterious (negative) effects observed. The field of study that explores these relationships is the field of Toxicology. We will use a few of their foundational ideas in our investigations and explorations within this course.
Dose: How Much
“Dose makes the poison” has become a guiding principle in toxicology. When determining whether a material is toxic, data on the route on ingestion (inhalation, ingestion, or contact with skin) and the dose relative to bodyweight are important qualifications to the assessment. In this section, we will briefly define terms to describe a lethal dose of a toxic substance.
Table 3 summarizes the toxicological definition of toxic and highly toxic, according to OSHA standards. A (median) lethal dose is abbreviated as LD50 and a median lethal concentration is abbreviated as LC50 (for toxic gases). Notice that highly toxic substances have a lower median lethal dose/concentration than toxic substances.
|
|
Administered Orallyα |
Continuous Contact with Skinβ |
Continuous Inhalation (in air)α |
|---|---|---|---|
|
Median Lethal Dose/Concentration |
LD50 (mg/kg bodyweight) |
LD50 (mg/kg bodyweight) |
LC50 (ppm) |
|
Toxic |
50 – 500 |
200 – 1000 |
200 – 2000; |
|
Highly Toxic |
<50 |
<200 |
<200 2 mg/L |
|
administered to: α albino rats (200-300 g); β albino rabbits (2-3 kg) |
|||
Duration: how long and how often[8]
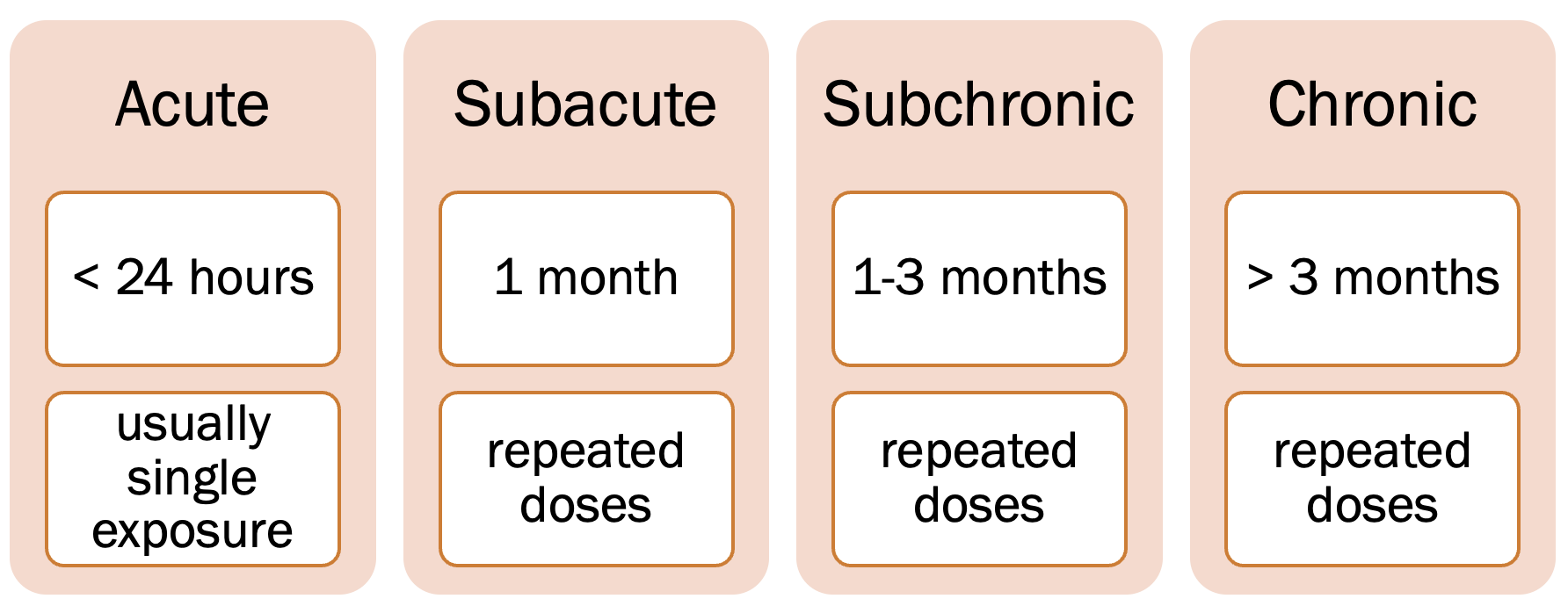
On the spectrum from acute to chronic toxicity, one goal of this course is to distinguish between the two extremes. Acute toxicity occurs less than 24 hours after exposure, usually a single dose but possibly multiple or repeated doses within a 24-hour period.[9] Chronic toxicity is the cumulative damage to specific organ systems over a period of greater than three months through repeated exposures (doses). Some substances—such as alcoholic beverages—can induce both acute toxicity (depression of the central nervous system) and chronic toxicity (cirrhosis of the liver).
M: Minimize the Risks of Hazards
Personal Protective Equipment (PPE)
Proper Attire (Required)
Students are required to wear lab-appropriate attire to be permitted in the laboratory:
- Tops: Shoulders, midriffs, and backs must be fully covered. Prohibited items include, but are not limited to, muscle tanks or other sleeveless tops, crop tops, backless shirts, and tops with mesh or other holey fabrics.
- Bottoms: Legs, including ankles, must be fully covered. If your pants are not quite long enough to cover your ankles, you must wear socks that are long enough to cover the exposed skin. Prohibited items include, but are not limited to, shorts, cropped pants (e.g., capris), jeans with holes, and sheer tights.
- Shoes: Feet, including toes, must be fully covered. If shoes do not completely cover the feet, socks must be worn. Prohibited items include, but are not limited to, sandals, flip-flops, and other shoes that are not well-secured to the foot.
Goggles (Required)
Students are required to protect their eyes with splash-proof safety goggles (OSHA-ANSI Z87.1-2010 standard or later), which can be purchased at the local bookstores serving MSU.
- Students who wear glasses should be sure to purchase goggles that fit over their glasses.
- Splash-proof safety goggles with indirect venting are recommended to reduce fogging.
- Under no circumstances should you wear contact lenses in the laboratory, even under goggles. Chemical vapors may dissolve in liquids covering the eye and concentrate behind the lenses. “Soft” contact lenses are especially dangerous as chemicals can dissolve in the lenses themselves and be released over a period of several hours.
Goggles should be worn at all times in the laboratory, unless told otherwise by a laboratory instructor. “At all times” includes periods when you personally are not experimenting; others may be conducting experiments, which still presents a hazard to others in the room. If you need to take off your goggles to adjust them or because they have fogged up, you should step outside the laboratory room to do so.
Gloves (Required)
Nitrile gloves are available in the laboratory in various sizes. You should wear the glove size that best fits your hands—too small, and the gloves are prone to tearing (leaving parts of your hand unprotected); too large, and you lose a great deal of dexterity (making it difficult to do fine tasks). If your size is not available, you can replenish the supply by taking the empty box to the stockroom. If there is no empty box corresponding to the size that you need, please alert your TA.
Wearing gloves at all times is not mandatory. However, for most experiments, such as those involving organic compounds, dyes or acids and bases, you are encouraged to wear them when working with these substances. Used gloves are disposable and shall be thrown to contaminated lab debris bin.
In order to avoid contaminating door handles or other surfaces, do not wear gloves outside the lab. If you must go into the hallway while handling something that requires a gloved hand, keep one hand ungloved, and use that hand to open doors. (Your TA may refer to this as the “one glove” rule.)
Lab Coat (Required)
Required laboratory attire includes a knee-length laboratory coat. Posters and guidelines on proper attire are available in CEM 255 laboratories. Here are some things to keep in mind:
|
P: Prepare for Emergencies
Preparation for emergencies involves proactive measures to minimize risk, plan for accidents and spills, and appropriate mediation measures. While your TA will be an invaluable resource in this preparation process, one key learning goal for the Planning Documents is to name the chemicals, the hazards they pose, and identify the major risks that your team will encounter and what necessary PPE will help mitigate that risk. If accidents (inevitably) occur, it is everyone’s responsibility to contain the accident.
On the first day of laboratory, your instructor or TA will point out the safety features of the laboratory classroom. These features are summarized below, and each teammate should be able to locate and operate with assistance these safety features. The locations of these safety features are approximated in the Figure 1 below.
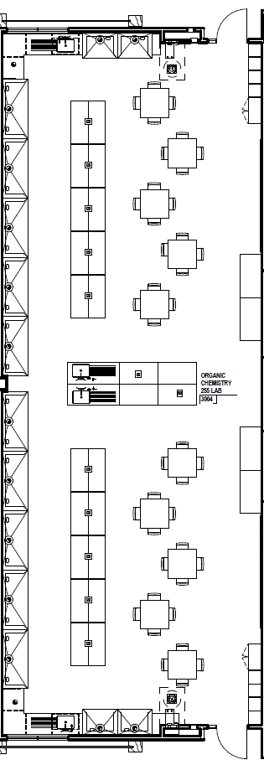
The waste (WH) and supply (SH) hoods are on the north and south ends of the classroom. The safety shower (SS) is just inside the door next to the waste hood and the eye wash stations (EW) are next to the supply hoods and the sinks on the north and south ends of the room. In the event that a chemical splashes near your eyes, use the station before the material runs behind your safety glasses and into your eyes. You should irrigate your eyes for at least five minutes and notify your laboratory instructor right away.
In addition to the general waste garbage cans throughout the space, there are glass waste boxes are available for broken glass. Never place broken glass in the general waste can. Be careful to never place your hand inside the glass waste box, and to never overfill this box. If glassware breaks on the counter or floor, consult your TA regarding any chemical contamination of the glass waste before using the broom to scoop up and deposit the glass waste in the glass waste box. Glassware that is broken in the sink should also be handled with care at the TA’s instruction. The final source of broken glassware is likely to be inserting glass tubing or thermometers into stoppers; to do so safely, lubricate both the tubing and the stopper hole with water. Wrap the tubing in a towel, grasp it as close to the end being inserted as possible, and push gently, using a twisting motion.
- Hill, R. H.; Finster, D. C. Laboratory Safety for Chemistry Students. 2nd edition, 2016. ↵
- ACS Chemical & Laboratory Safety. “Basics & RAMP” ACS Institute Safety Basics & RAMP ↵
- ACS Chemical & Laboratory Safety. “Basics & RAMP” ACS Institute Safety Basics & RAMP ↵
- American Chemical Society. Free Online Course: Foundations of Chemical Safety and Risk Management. American Chemical Society Login ↵
- National Fire Protection Association. NFPA 704: Standard System for the Identification of the Hazards of Materials for Emergency Response. NFPA 704 ↵
- UNECE. Globally Harmonized System of Classification and Labelling of Chemicals, 2015. UNECE GHS ↵
- Interactive Learning Paradigms Incorporated (ILPI) “The MSDS Hyperglossary: Toxic” 11 Nov 2020 MSDS Hyperglossary: Toxic ↵
- Nardei, S. “Toxicology Basics” from University of Arizona Southwest Environmental Health Sciences Center. Toxicology Basics ↵
- Beyond Benign. “Introduction to Toxicology” Beyond Benign: Introduction to Toxicology ↵


