Part 7: Assignment Guides
25 Planning Documents
Each session your team will plan the experiments to be conducted the following session. This should be completed as a team with the recorder, ensuring that the information is submitted on D2L. During the planning phase, the communicator needs to summarize the plan and gain approval from your TA as the plan is being developed so they make sure you are not going too far off task or planning to do something dangerous or impossible.
Your TA is not there to give you procedures or tell you if you are doing it “right”. The main focus of this lab is about using your chemical knowledge and critical thinking skills to solve and report on problems; this laboratory is less focused on having the right answer at the end of the day. It is up to each member of the team to research the problem before the lab period and come prepared to work with the team to plan a solution to the project scenario. Each part of a project has guiding questions to help guide your team’s thinking. These questions are only part of the information needed in the planning phase, as indicated in the Planning Document rubric.
Time management and properly dividing up work during the planning stage is essential for success in this course. While planning is done as a group, each person is expected to work independently and conduct complete experiments. Each lab period is 2 hours and 50 minutes long. If you work as a single entity and conduct experiments together, that is all of the time you have. But if each member works independently to conduct a well-planned procure, that amount of time is quadrupled allowing your team to accomplish much more in the same amount of physical time! If each team member cannot independently collect data to analyze that feed into the project, then the work is not organized correctly. Note that this does not mean that each person must solve all the project problems independently; it means that each person is making an important contribution to the project goal(s).
Ideally your project plan should have several sections:
- Responses to planning questions. Based on the project scenario, you will have to answer questions about relevant background information, what project goals you will focus on, and/or ideas about how to approach the goals. You should cite the references you used in responding to these questions.
- Equipment and chemical lists. List all chemicals (and hazards using SDS info) that you will be using. Provide a list of equipment needed outside of the routine equipment in your drawer and include a drawing of the apparatus setup for this project.
- Equations. Include both chemical and non-trivial mathematical equations you will be using, if applicable.
- Detailed procedure for each person! Each team member should have their own set of experiments that they can work on independently of the group. Everyone needs to be doing their own small slice of the project that will contribute to the overall project goals. Everyone has a purpose and each person is doing their own experiments. No one is measuring things out for the whole group, cleaning up after the whole group, or just observing. Everyone does meaningful work!
- Include as much detail as possible. This will hold everyone accountable and provide each member a list of actionable items and techniques so that each person knows what they need to know and do for the next lab period.
Planning documents for the Identification of an Unknown Organic Solid and the Synthesis and Analysis of Painkillers were provided as examples in the Appendix.
Planning Document Template
Title of Lab
|
Team Leader |
Recorder |
Communicator |
Safety Officer |
|---|---|---|---|
|
|
|
. |
|
Planning Questions
Chemicals
|
Chemical Name |
Hazards |
Personal Protective Equipment (PPE) |
|---|---|---|
|
|
. |
|
Waste Management
|
Disposal Method |
Material(s) or Chemical(s) That Should Be Disposed By This Method |
|---|---|
|
Sink |
|
|
Trash Can |
|
|
Waste Carboy |
|
|
Other (Specify) |
|
Equipment
Equations (Reactions, Equations, or Green Metrics)
Project Plan (Procedures)
Write a preliminary plan for your experimental procedure. Indicate what each person in your team will do to solve the problem and what data they will record. Remember, this is not the team roles. Break down the work so you use your time effectively and that each person will end up with a mini lab report for their data and notes assignment next session.
Sample Planning Document
Investigation of Luminol Reaction
|
Team Leader |
Recorder |
Communicator |
Safety Officer |
|---|---|---|---|
|
Teammate 1 |
Teammate 2 |
Teammate 3 |
Teammate 4 |
Planning Questions
Responses to planning questions go here.
Chemicals
|
Chemical Name |
Hazards |
Personal Protective Equipment (PPE) |
|---|---|---|
|
Luminol |
Not classified as hazardous |
Handle with gloves |
|
Bleach (sodium hypochlorite, NaClO) |
Hazardous Skin corrosive – Cat. 1 Serious eye damage – Cat. 1 |
Handle with gloves |
|
Copper sulfate (CuSO4) |
Hazardous Acute toxicity, oral – Cat. 4 Skin irritant – Cat. 2 Eye irritant – Cat. 2 |
Handle with gloves |
|
1 M sodium hydroxide (NaOH) |
Hazardous Skin corrosive – Cat. 1 Serious eye damage – Cat. 1 |
Handle with gloves |
Waste Management
|
Disposal Method |
Chemical(s) That Should Be Disposed by This Method |
|---|---|
|
Sink |
Excess NaOH (after neutralization) |
|
Trash Can |
|
|
Waste Carboy |
All reaction mixtures; excess luminol, bleach, and copper sulfate |
|
Other (Specify) |
|
Equipment
- Small beakers (1 per team member)
- Small test tubes
- 10 mL graduated cylinder (to make solutions)
- Dark container (to better see reaction progress)
- Stopwatch (to time reactions)
Equations
- Luminol reaction (reference link: Chemiluminescence of luminol: a cold light experiment)
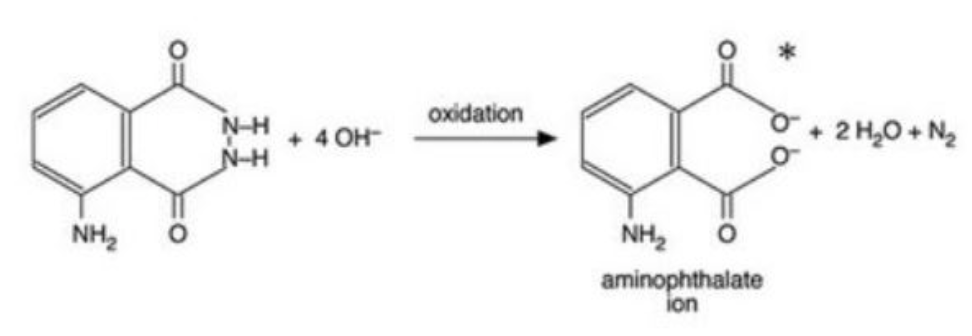
Project Plan (Procedures)
Teammate 1
Goal: Determine the effect of bleach on the rate of luminol reaction.
Procedures:
- Dissolve 10 mg of luminol in 2 mL of 1 M NaOH solution in a small test tube.
- In small beaker, mix 0.9 mL of bleach with 9.1 mL of water.
- To start the reaction, add 1 mL of bleach solution to the luminol solution (start timer as soon as mixed).
- Observe reaction in a dark box and time the length of reaction.
- Repeat the above procedure using the following bleach solutions:
|
Bleach (mL) |
Water (mL) |
|---|---|
|
1.8 |
8.2 |
|
3.6 |
6.4 |
|
5.0 |
5.0 |
|
10 |
0 |
Data Analysis: Plot length of reaction (time) on y-axis and % bleach on the x-axis. See if there is a trend.
Teammate 2
Goal: Determine the effect of luminol on rate of luminol reaction.
Procedures:
- Dissolve 4 mg of luminol in 2 mL of 1 M NaOH solution in a small test tube.
- In a small beaker, mix 0.9 mL of bleach with 9.1 mL of water.
- To start the reaction, add 1 mL of bleach solution to the luminol solution (start timer as soon as mixed).
- Observe reaction in a dark box and time the length of reaction.
- Repeat the above procedure using 6 mg, 8 mg, 10 mg, and 12 mg of luminol dissolved in 2 mL of 1 M NaOH Solution.
Data Analysis: Plot length of reaction (time) on y-axis and mass of luminol on the x-axis. See if there is a trend.
Teammate 3
Goal: Determine the effect of CuSO4 catalyst on rate of reaction.
Procedures:
- Dissolve 10 mg of luminol in 2 mL of 1 M NaOH solution in a small test tube.
- Add 1 mg of CuSO4 and stir to dissolve.
- In a small beaker, mix 0.9 mL of bleach with 9.1 mL of water.
- To start the reaction, add 1 mL of bleach solution to the luminol solution (start timer as soon as mixed).
- Observe reaction in a dark box and time the length of reaction.
- Repeat the above procedure using 2 mg, 3 mg, 4 mg, and 5 mg of CuSO4.
Data Analysis: Plot length of reaction (time) on y-axis and mass of copper sulfate on the x-axis. See if there is a trend.
Teammate 4
Goal: Assist other group members during their trial and ensure every team member follow safety guidelines.
Data Analysis: Summarize the data everyone collected and propose a solution.
Planning Document Success Guide
This rubric will be embedded into D2L for the lab instructor to evaluate your plan. They will provide you feedback in the comment portion on D2L of any strengths to your plan and any changes that your team needs to make. Planning documents are 10 points total, graded holistically, with an emphasis on the procedures and safety.
|
Goals for Planning Document |
Strengths & Suggestions for Improvement |
|
|---|---|---|
|
Planning Questions |
Did the team address the planning questions and incorporate the responses into their plans? |
|
|
Chemicals |
Did the team consider the hazards and risks of all the chemicals needed? |
|
|
Waste Management |
Did the team designate the waste management plan for all chemicals and materials? |
|
|
Equipment |
Did the team include an equipment list, especially including items not found in their team drawers? |
|
|
Reactions & Equations |
Did the team make note of the reaction (if synthesizing) or any equations/green metrics they will need? |
|
|
Procedure |
|
|

