Part 4: Setting Up the Reaction
12 Monitoring a Reaction
How to Monitor a Reaction by TLC:
Find a Suitable Elution Solvent System for Your Reaction
The TLC chamber needs to be set up and ready to go before you start your reaction. Find a suitable solvent system that will make your starting material run to about Rf = 0.5 (or half-way up the plate). If this is the first time you have dealt with this compound, here are a few tips that will make it easier to find a suitable system:
- If the compound is polar (for example, an alcohol), you will probably need a fairly polar solvent system. For nonpolar compounds, a nonpolar solvent system is necessary.
- Do at least two solvent system trials at a time using solvents of different polarities.
- You may need to use mixed solvent systems to get a good Rf—many compounds can be separated by using some mixture of ethyl acetate (polar) and toluene (nonpolar), for instance. The solvents you choose in a binary or multi-solvent system must be miscible.
- Remember, it doesn’t matter what you dissolve the starting material in—it will evaporate (unless you use water) so you should use the solvent with the lowest boiling point possible to dissolve the analyte. Dissolving solvent and elution solvent can be the same or different solvent (systems).
Remove a Sample
You can do this by taking a small aliquot from the reaction vessel with a capillary tube. You do not need much; a dip and drop is plenty.
Prepare the Sample for TLC
Make sure that you are sampling only the organic components of your reaction mixture. If your reaction is in an organic solvent with no inorganic materials present, you can spot the reaction mixture directly onto the TLC plate.
If, however, you have water, some other very polar solvent with a high boiling point, or inorganic materials present, you will perform a microscale work-up of the small sample you have taken.
Provide Comparison Samples on the TLC Plate
Thin layer chromatograms (TLCs) are difficult to replicate for beginners. The Rf value and TLC result of a particular analyte can vary considerably depending on the concentration of the spotting solution, the size of the spots, the temperature, the vapor pressure inside the developing chamber, and so on.
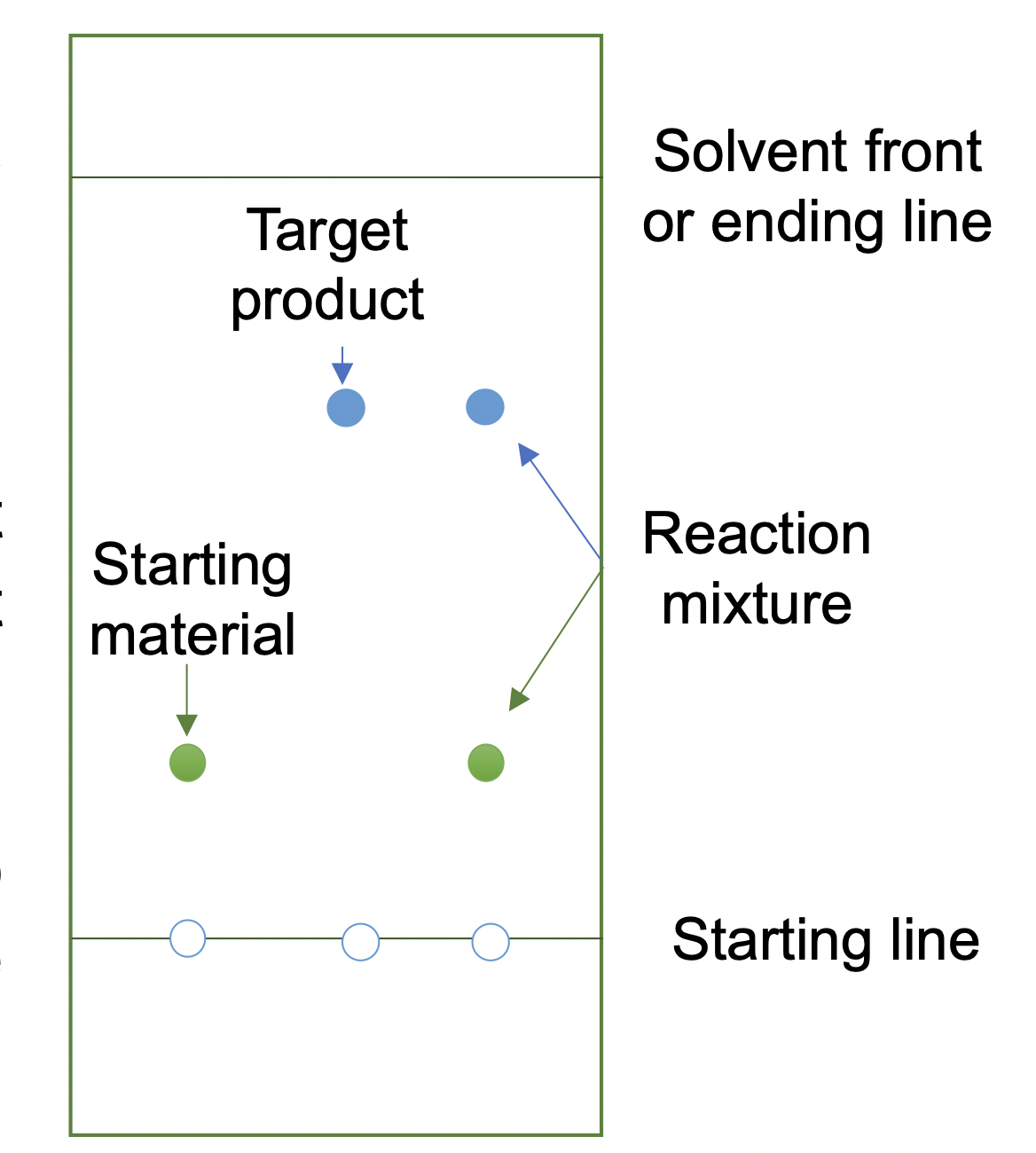
This lends itself to a more qualitative comparison in which you run the sample side-by-side with the starting material on the same plate. It is a good idea to always spot the starting material on the same place on each plate, such as on the left, so that if you were to forget to label the compounds you would still know what each spot was.
It is also a good idea to spot the starting material, target product and reaction mixture on the same TLC plate for verification of each compound and monitoring reaction progress.
The starting line you draw must be above the solvent level in the TLC developing chamber. After you put the TLC plates into the TLC chamber with the bottom of the plate in contact with the elution solvent, you will see the solvent front rising along the plate due to capillary effect. Stop the TLC analysis when the solvent front is approximately 1 cm away from the end of the plate, or when you predict there is good separation already.
As you follow the reaction by TLC (roughly 15-minute intervals), you should see a new spot appear as the product is formed and the relative amount of the starting material should decrease. If more than one product is possible, yet you only desire one of the products, you may be able to stop the reaction when you see the formation of the secondary product.
Not all compounds will show up on TLC under UV-visible light. More information on TLC can be found in the next section.

