Part 3: Glassware & Laboratory Equipment
7 Measuring Devices
Measuring Liquids by Volume
Liquids can be measured either by weight or by volume. It is often more convenient to measure the volume of a liquid and then convert it to mass if the density is known (remember, density = mass/volume, with unit).
| Image of Glassware | Name and Description of Glassware |
|---|---|
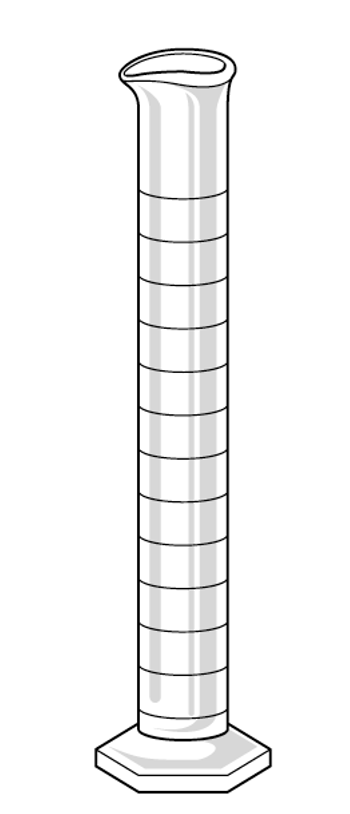 |
Graduated Cylinders
|
Volumetric Glassware
Volumetric glassware is used when great accuracy is required, for example, during titrations, or when making up standard solutions of known volume.
| Image of Glassware | Name and Description of Glassware |
|---|---|
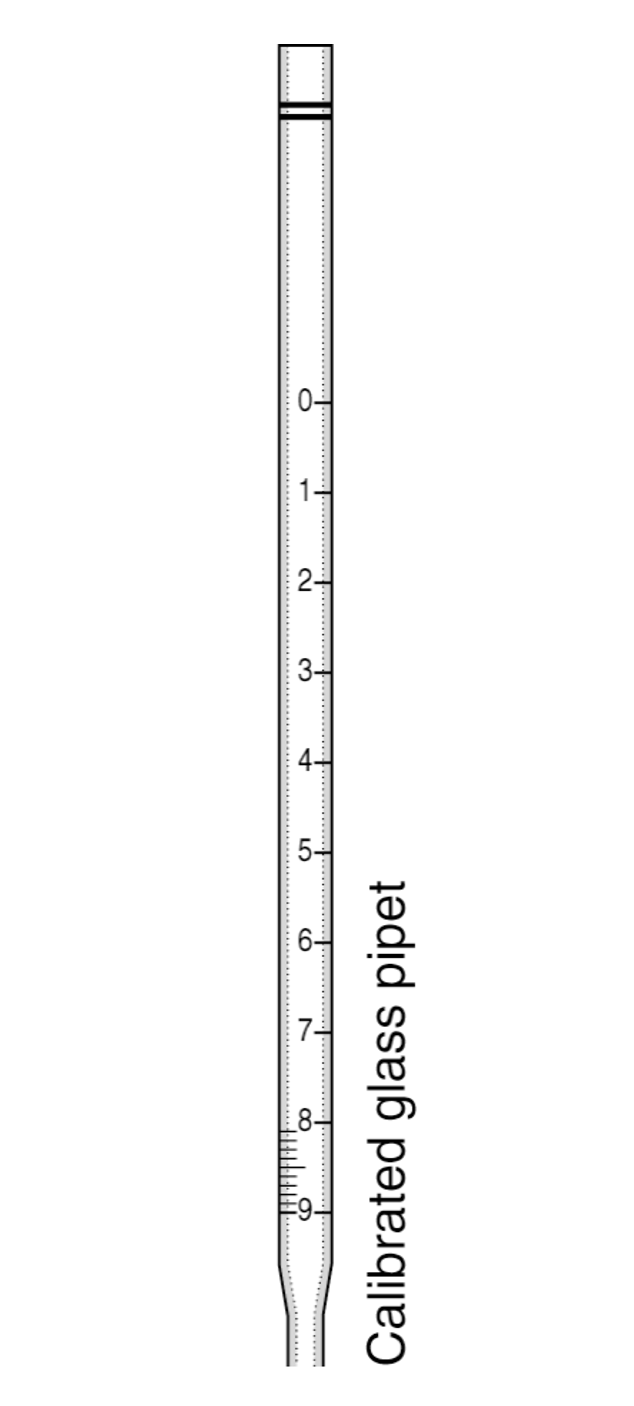 |
Pipets
Safety note: Never pipet by mouth. Other note: Use the appropriate number of significant figures based on the precision of the measurement.
|
Measuring Solids and Liquids by Mass
Laboratory Balance
A laboratory balance is used to obtain the mass of various objects. We have two different types of electronic balances in the lab:
- Analytical Balances are accurate to 0.0001 g (a tenth of a milligram) and are used to weigh out samples of less than 1 gram as the high accuracy results in low capacity.
- Precision Balances are accurate to 0.001 g (a milligram) and are used to weigh out samples over 1 gram.
In this course you will likely use the precision balances. These balances are accurate to 0.001 g and simple to use, but they are delicate and expensive. Please treat them with care and respect and leave them clean. If you spill anything solid on the balance, use the attached brush to clean it. If you spill a liquid, notify the stockroom personnel immediately.
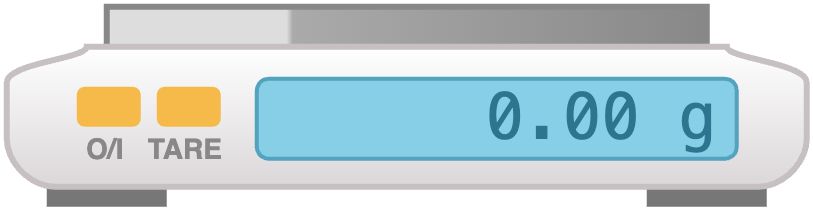
Note: The balances are fitted with draft shields to remove fluctuations of the readings. Please do not remove them.
Use of Laboratory Balances
The video Weighing Using An Analytical Balance provides an overview of using either type of balance (even though it talks only about the analytical type balances). The step-by-step instructions begin at approximately 1:09.
- Never weigh anything directly on the balance. Always use a weigh boat, beaker (for highly corrosive compounds), or some other container to contain the chemical.
- Never pour anything into a container in the balance. You should always remove the container to add or remove material. This should help keep the balance clean.
- Never leave the balance dirty. If you should spill something in the balance, be sure to clean it out. If you are not 100% sure how to do so, ask your instructor.

