Part 5: Organic Laboratory Techniques
14 Heating and Cooling
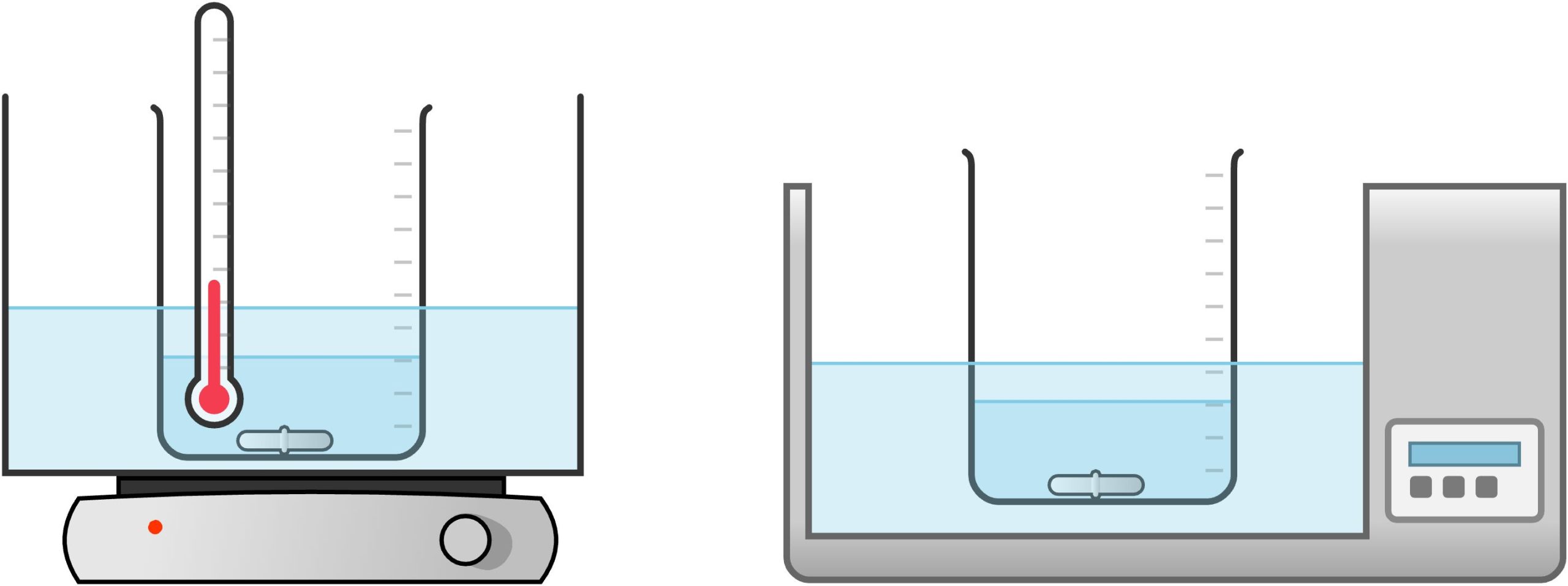
Heating Bath
It is often necessary to heat reactions or solutions in a controlled manner so that the temperature does not exceed a certain value. For example, many reaction mixtures might decompose when heated to very high temperatures. One way to avoid this problem is to use a water bath. As noted in the Suggested Reaction Setups, you should consider a heating mantle (if available) or make use of water, oil, or sand baths, in which a large glass dish is filled with an appropriate medium that is heated either on a hot plate or with an immersion heater. These methods allow for controlled heating and an easy way to monitor the temperature.
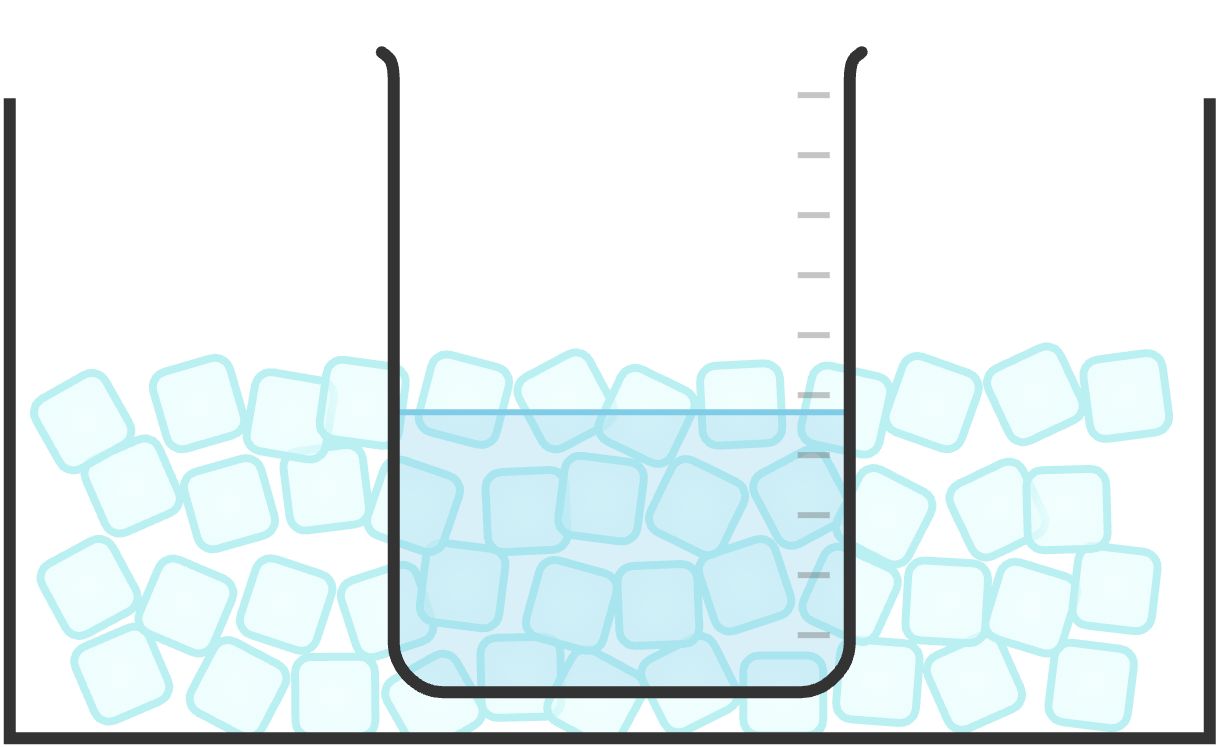
Cooling Bath
A cooling bath is used to cool a reaction or system under investigation.
Water-and-ice mixtures can reach temperatures as low as 0 °C.
For temperatures lower than 0 °C, a mixture of ice, water, and salt can be used to achieve a temperature of about -10 °C.
It is unlikely that you will need to reach a temperature even lower than -10 °C in the organic chemistry lab, but it is possible to do so using dry ice (solid carbon dioxide) in organic solvents.
Measuring Temperature
Thermometer
For most purposes, the classic thermometer is suitable for measuring temperature. Scientific thermometers typically measure temperature using the Celsius (°C) scale. Our course will make use of Vernier Temperature Probes instead.
Vernier Temperature Probe
The stockroom has temperature probes that connect to your computer via USB. This allows you to monitor and record temperature in Vernier’s LoggerPro software. This is especially useful if you want to record temperatures over time (i.e., make a temperature vs. time plot). By recording the temperature this way, it is easy to determine when a maximum or minimum temperature was reached, for example, during a chemical reaction or when a temperature stabilized for a certain amount of time.
Note: The majority of this chapter has been adapted from the CEM 161/162 manual: Cooper, M. M. et. al. Cooperative Chemistry for Michigan State General Chemistry Laboratories, 2019.

