Part 7: Assignment Guides
28 Formal Project Report
What is a Formal Report?
Scientists are investigators who "try out" ideas: they conduct experiments to provide evidence for or against these ideas. They share the results of their experiments in papers and other reports to allow others to learn the results of their investigations. You, too, will conduct experiments, record observations, collect data, and formulate evidence-based conclusions. For one project in this course, you will be asked to write a formal report describing the experiment, summarizing your results, and explaining your conclusions or judgments about the meaning of what you observed.
Audience
Professional scientists write for various audiences: themselves, their colleagues, or the public. They must write in a manner appropriate for the chosen audience. In preparing your lab report, think of the reader as an educated person who is interested in learning about your experiment; for example, you might imagine that you are trying to describe and explain your work to an organic chemistry student at a different institution who has not done this project. Although you know your reader will be your TA, who knows quite a lot about your experiments, you are the expert on what you did. It is your responsibility to communicate what you did and what it means efficiently and accurately.
Scientific Style
As you write, pay close attention to the “style” of your work. In scientific papers, facts and interpretation of those facts are what counts; thus, depersonalized writing known as "scientific" or "academic" is most appropriate for your lab report. What are the common elements of this style?
- Use the third person and passive voice. Although you are describing what you did, scientific reports should be impersonal. The easiest way to do this is to use the third person and passive voice (“X was done to Y,” which contrasts with active voice: “X did Y”). First person (“I/we/me/us”) is generally discouraged, and second person (“you”) should not be used.
- Use the past tense. You are writing about the experiment you have already completed; therefore, everything you describe happened in the past.
- Be as accurate and specific as possible. Successful scientific description requires exact detail.
- Be clear and concise. Scientific reports are not the place for flowery prose or roundabout descriptions.
Let’s look at some examples:
|
YES/NO |
Example Text |
Comments |
|---|---|---|
|
NO |
Student M isolated the product using vacuum filtration. |
It does not matter who did it, just that it was done. |
|
NO |
Isolate the product using vacuum filtration. |
You should tell the reader what you did, not tell the reader to do it (i.e., use declarative sentences, not imperative sentences). |
|
NO |
A side-arm flask, Buchner funnel, and filter cone were obtained. Thick-walled tubing was connected to the sidearm flask fitted with the Buchner funnel. Filter paper was placed in the funnel and moistened with a small amount of water. Vacuum was achieved via water aspiration. The reaction mixture was poured into the funnel, thus isolating the solid product from the rest of the solution. |
This is too much detail. Typically, common techniques do not need to be described in step-by-step detail; you just need to be clear about what technique you used. Unless you modified a standard procedure or did something novel, then you do not need to get into individual steps of how the technique was performed. |
|
NO |
The product was isolated. |
This is not enough detail. There are many ways to isolate a product. What method did you use? |
|
YES |
The product was isolated by vacuum filtration. |
This gets straight to the point of how you collected a reaction product. |
The style used in scientific reports may be quite different from that required for a paper in another field. It is important that you write in a style appropriate for your audience, which may require you to understand and get used to using different writing styles.
General Format
The following is an outline of the fundamentals of writing a lab report. Please read it carefully. There is also a sample laboratory report with comments for you to read. The grading criteria for your lab reports will refer to the following outline.
Lab reports contain the following sections: Title Page, Introduction, Experimental Methods, Results, Discussion, Conclusion, and References. Your lab report must be typed and should include tables and figures (e.g., graphs, molecular structures, etc.) where necessary. The accepted font is Times New Roman, size 12, and the pages must be double-spaced.
Title Page
The first page of the report is the title page. The title should reflect the content and focus of the project described in the report. It should be as short as possible and include essential key words.
Note: Due to MSU Regulations concerning the use of Turnitin.com, do not put your name on the title page. The D2L System will manage the file and ensure we know who turned in what paper.
Introduction
The text of the report begins with an introduction. In this section of the paper, concisely tell the reader what you intended to do and why it was important to do it. Make sure you clearly communicate to the reader the purpose of the experiment(s) and provide background information required to understand the experiment(s) and the reason it was worth doing. Remember, longer is not necessarily better; indeed, for your report, you will likely need no more than one page to provide a suitable introduction.
You must cite credible sources to support statements you make about the scientific background or basis for your investigation. Citations tell the reader a few things: 1) that you have prepared properly for the experiment by providing yourself with a relevant background from experts in the field or other accepted authorities, and 2) where they can delve more deeply into the subjects you have introduced.
These citations should be numbered consecutively in the text and listed in the References Section. Typical sources for your citations include textbooks, your lab manual, reference books, and internet resources. For details on formatting your citation, see References at the end of this section.
Experimental
In this section, you give the reader a detailed account of the actual experiment. Scientific experiments are not considered valid unless they can be repeated by others. In this sense, science has no secrets: scientific theories become established only when the experiments that led to them can be repeated or verified by others besides the original investigators.
Scientists also use lab reports as a means of learning and sharing techniques. While others may not choose to duplicate your exact experiment, they may choose to use your procedure in a similar investigation. Thus, the experimental section of your lab report should be sufficiently detailed such that others could read your lab report and successfully duplicate your experiment.
Results
In this section you summarize the outcome of your experiments for your reader. This section will consist primarily of data that you and your group members gathered in the course of the experiment. You must organize the data so that it is easy to read. Numerical data is usually presented in tables. Relationships between sets of data (e.g., intensity vs. wavelength) are usually presented as graphs. Graphs, drawings, and other images are called figures.
All tables and figures should be numbered and labeled descriptive titles (e.g., Figure 1: Infrared Spectrum of Polypropylene). Any tables or figures should also be introduced in the written body of your report, using its corresponding number (e.g., “The infrared spectrum of polypropylene is shown in Figure 1”). Place tables and graphs near where you mention them in the body of your report. This makes it easy for the reader to use and understand the graphs, charts, tables, etc.
Discussion
The discussion section of your report is the most important one for you and your reader. In this section of the report, you interpret the results of your experiment for the reader. You explain what the results mean (claim), how you know what they mean (evidence), and why your claims make sense (reasoning). Here, you will also mention any weaknesses or limitations of the methods you used. This demonstrates not only how successful your experiment was but also how well you understood the experiment. The discussion section can be difficult to write, but you will learn more about your experiment and yourself as an investigator as you write it.
Before you begin writing this section, review your Introduction, Experimental, and Results sections. Look at all these sections, as well as your lab notebook, as you write the Discussion section. In the context of the goals you presented in the Introduction, you should discuss all of the results of all of the experiments that you presented.
Conclusion
Your overall conclusions about the project have probably already been mentioned elsewhere in your report. You may have predicted some of the outcomes of your experiments in the Introduction and discussed them again as an empirical conclusion (meaning that it was derived from your experiments and observations) in the Discussion section. In the Conclusion section, you should briefly summarize those major conclusions. In a sense, it is a summary of the Results and Discussion sections combined. A single paragraph may be enough to clearly summarize the outcome of your investigation.
References
The final section of your report tells the reader where to find any of the sources of information you used in your report. In the body of your report (particularly in the Introduction and Discussion sections), you will have mentioned other sources of information. Each of these references should be numbered consecutively within the text as superscripts. At the end of your report, include a complete reference list, numbered in the order in which you mentioned each source. The reader can use this list to follow up on any source you mentioned or to do additional reading.
References should follow the American Chemical Society Guidelines for superscript number format, which can be found in the ACS Style Guide.
Here is an example:

Format for Typical References
|
Source Type |
General Format |
Example |
|---|---|---|
|
Journal Article |
Author (Last Name, Initials). Journal Title Abbreviation (italics—see ACS Style Guide page 288 for abbreviations), Publication Year (bold), Volume Number (italics), starting page number—ending page number. |
Carmel, J.H.; Herrington, D.G.; Posey, L.A.; Ward, J.S.; Pollock, A.M.; Cooper, M.M. J. Chem. Ed., 2019, 96, 423—434. |
|
Book |
Author (Last Name, Initials), Book Title (italics), Publisher: City, Publication Year (bold), page number. |
Harte, J. Consider a Spherical Cow. William Kauffmann, Inc.: Los Altos, CA, 1985, 83—88. |
|
Web Page |
Web Page Title. URL (accessed Mon. Day, Year). |
Journal of Chemical Education (ACS Publications). https://pubs.acs.org/journal/jceda8 (accessed Apr. 10, 2019). |
|
Course Lab Manual |
Author (Last Name, Initials). Title of Manual. Institution Name, City, State. Student laboratory manual, Year. |
Cooper, M.M.; Day, E.L.; Zhang, M. Cooperative Organic Chemistry Student Laboratory Manual. Michigan State University, East Lansing, MI. Student laboratory manual, 2024. |
|
Your Lab Partner’s Work |
Author (Last Name, Initials). Institution Name, City, State. Personal communication, Year. |
McStudentface, S. Michigan State University, East Lansing, MI. Personal communication, 2019. |
Plagiarism and Turnitin
What is Plagiarism?
Examples include direct copying of someone else’s work, published or unpublished, and representing it as your own; sentence-by-sentence paraphrasing of someone else’s work; changing words here and there in material from another source. If you use a reference or another source, you should cite it and convey the ideas that you are using from the reference in your own words. In short, make sure that all ideas in your work for this course are expressed in your own words. It is only when you are able to express scientific ideas using your own words that you truly understand what you are writing about. In addition, figures (diagrams, graphs, etc.) and tables submitted for an individual grade must be your own work. Make it clear which ideas are your own and which came from other sources. Whether accidental, blatant, or self-plagiarism, the same standards and penalties apply. Additional information about plagiarism and MSU’s policies on plagiarism can be found on the website of the Office of the Ombudsman.
Anything submitted for an individual grade, even though such assignments will be based on your team’s experimental work, must be your own work and not copied from your fellow team members. Do not share your work on the Formal Report assignments with other students or ask other students to see their work on Formal Report assignments because both constitute academic misconduct for these assignments.
Use of Turnitin
The following has been adapted from “Syllabus FAQ,” Office of Ombudsman.
Consistent with MSU's efforts to enhance student learning, foster honesty, and maintain integrity in our academic processes, we have chosen to use a tool called Turnitin to compare your papers with multiple sources. The tool will compare each paper you submit to an extensive database of prior publications and papers, providing links to possible matches and a “similarity score.” The tool does not determine whether plagiarism has occurred or not. Instead, we will make a complete assessment and judge the originality of your work. All submissions to this course may be checked using this tool.
You should submit papers to Turnitin via D2L without identifying information included in the paper (e.g., name or student number). The D2L system will automatically show this information to us when we view the submission, but the information will not be retained by Turnitin. If you forget and submit your paper with your identifying information on it, your identifying information will be retained in the Turnitin repository. Your submissions will be retained in the Global Turnitin repository.
In choosing to use Turnitin in this class, we have agreed to follow five guidelines:
- We will use Turnitin as part of a balanced approach to encourage academic integrity and foster student success.
- We will openly disclose use of Turnitin in this course on the syllabus and at the time assignments are announced.
- For a given assignment, we will use Turnitin for all papers.
- We will make the final determination of originality and integrity.
- To ensure privacy, we will ask students to remove identification (e.g., names and student numbers) from submissions.
If you have any questions about the use of Turnitin in this course, please bring them to your instructor’s attention.
Formal Report Success Guide
Your lab instructor will read, comment on, and evaluate your report. They will comment on how well you communicate necessary background information as well as the processes and results of the experiment itself. Your instructor also observes how carefully you follow the format for lab reports described in this manual. The guide included here is a general guideline and shows how your report will be assessed. Within each section, the instructor will comment on the strengths of your writing and offer suggestions for improvements you can make. Make sure you understand what the goals on the guide mean. Read them carefully and ask your lab instructor about any which puzzle you.
You will be submitting your formal report within one week after the last lab section.
Introduction Guideline
|
Introduction |
||
|---|---|---|
|
Goals for Introduction Section |
Strengths & Suggestions to Improve |
|
|
Introduction of scenario and project goals |
Does the introduction summarize the problem to be investigated? Does the introduction highlight the relevant project goals and/or criteria for answering the driving question posed in the scenario? |
|
|
Relevant and sufficient background |
Does the introduction include—as needed—any relevant background information that sufficiently supports choice of experiments? |
|
|
Originality |
Are ideas from the project scenario or relevant background information expressed in their own words and organization? (Paraphrased and summarized, not verbatim) |
|
|
Reference use |
Does the introduction appropriately use and cite outside literature? |
|
Experimental & Results Success Guide
Experimental |
||
|---|---|---|
|
Goals for Experimental Sections |
Strengths & Suggestions to Improve |
|
|
Original description of methods |
In the experimental section, are the procedure(s) that the team used described in their own words? (i.e., not just copied from the project scenario) |
|
|
Basis in literature |
For experimental details that were incorporated based on external reference materials, were these references appropriately cited? |
|
|
Clear & reproducible |
Is the procedure provided completely clear and detailed? From this section, could a peer replicate the entire experiment? |
|
|
Experiment details only |
Does not mention any details that belong in other sections (i.e., provides experimental details only). |
|
|
Procedure format and organization |
Is the section formatted as a narrative (paragraphs)? Is the section logically organized (by experiment)? |
|
Results |
||
|---|---|---|
|
Goals for Results Section |
Strengths & Suggestions to Improve |
|
|
Inclusion of important results |
Does the section include all results (positive AND negative)? |
|
|
Results only |
Is this section focused on presentation of the results? (Discussion, analysis, or interpretation of results in the next section) |
|
|
Presentation |
Are the data presented in an appropriate, easily readable format? Are all measurements labeled with appropriate units? |
|
|
Graphs & data tables |
Do all graphs and tables include appropriate labels and titles? |
|
Discussion & Conclusion Success Guide
Discussion |
||
|---|---|---|
|
Goals for Discussion Section |
Strengths & Suggestions to Improve |
|
|
Claim |
Does the data presented relate to the claim made? |
|
|
Evidence |
How strongly does the evidence support the claim? Are all relevant pieces of evidence included? |
|
|
Reasoning |
Does the Discussion provide scientific reasoning that fully explains how the claim and evidence are connected? Does the reasoning demonstrate an understanding of the relevant scientific principle(s)? |
|
|
Relates results to project goals |
Does the Discussion of results clearly and logically relate to achievement of project goals? |
|
|
Discussion of error and/or limitations of experiments |
Does the Discussion identify unexpected or inconsistent results? Does the Discussion describe possible sources of error? If no unexpected results are observed, does the Discussion describe the limitations of experimental approach or methods? |
|
Conclusion |
||
|---|---|---|
|
Goals for Conclusion Section |
Strengths & Suggestions to Improve |
|
|
Project Goals |
Are all of the goals for the project addressed? If applicable, do they suggest future work based on their results? |
|
|
Limited to summary |
Is the conclusion a concise summary with no new data presented? |
|
References |
||
|---|---|---|
|
Goals for Reference Section |
Strengths & Suggestions to Improve |
|
|
Complete source list |
Are all the cited sources in the paper listed? Does the list contain at least one source beyond the course materials? |
|
|
In-text citations |
Overall, are the In-text citations in proper superscript numeric format AND numbered in order of appearance? |
|
|
Reference list format |
Are the references in ACS style? |
|
Overall |
||
|---|---|---|
|
Goals for Overall Writing Presentation |
Strengths & Suggestions to Improve |
|
|
Clarity and precision |
Is the argument presented in the overall report completely clear and precise with adequate detail? |
|
|
Units |
Are all of the measurements labeled with appropriate units? |
|
|
Logical and readable |
Are all the sections logical and easy to follow? |
|
|
Style |
Is the entire report written in scientific style? |
|
|
Title |
Is the title page correctly formatted? |
|
How to Submit Your Report
Accepted File Formats
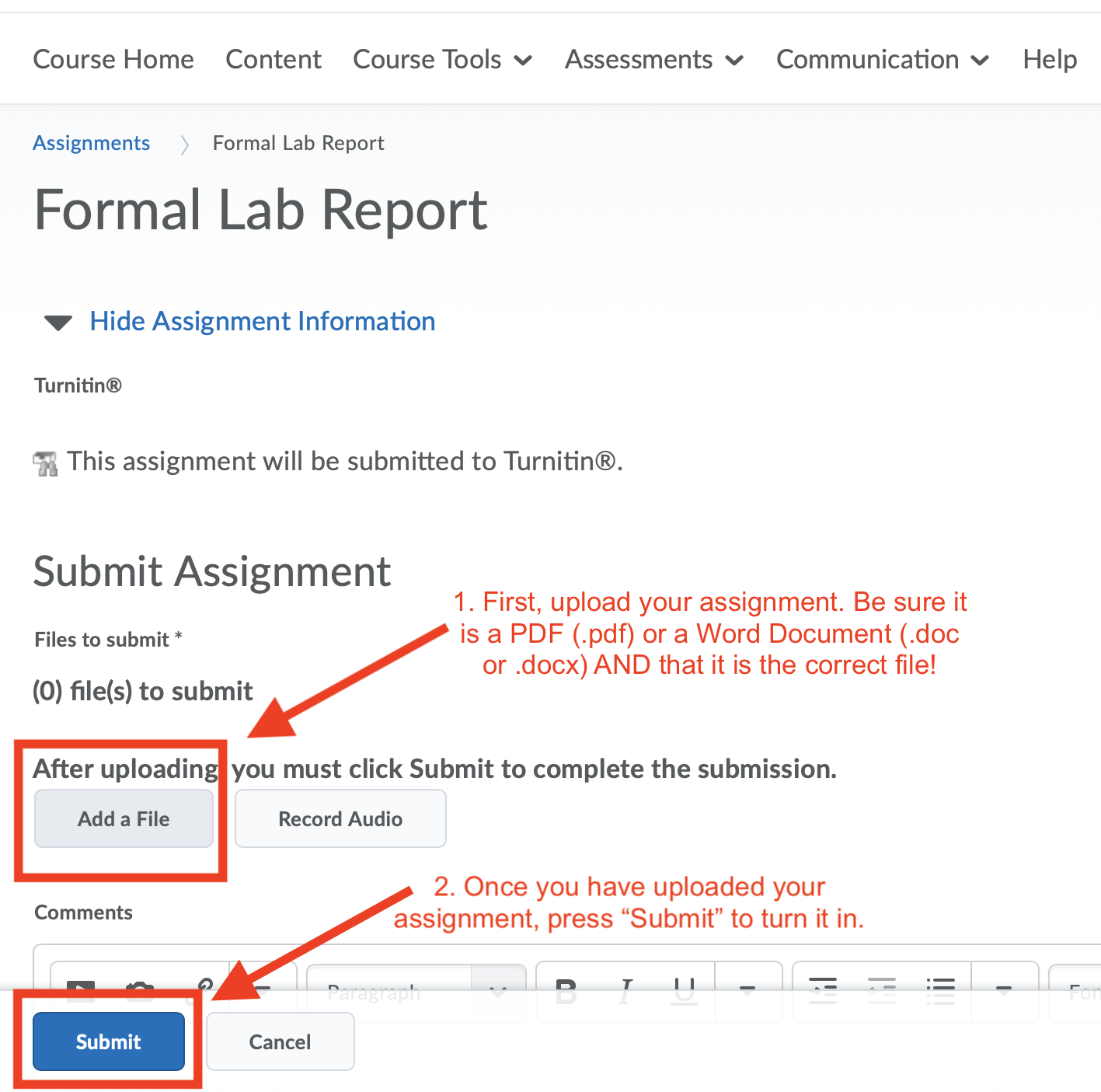
You can use any word processing program you wish: Microsoft Word, Apple Pages, Google Docs, etc. However, regardless of what program you use, you must submit your file as a PDF (.pdf, preferred) or Word Document (.doc or .docx) format. Other formats, including Apple Pages (.pages) documents, cannot be read by Turnitin. Therefore, you should be especially careful if you use a program other than Microsoft Word since the default file type is likely not a PDF or Word Document. Most programs can save files as PDFs, which is the preferred file type for Turnitin.
Note: The file must include the .pdf, .doc, or .docx extension in its name.
If you need to manually add the extension to the file name, or you are unsure if the extension is in the file name, consider the steps below:
- MacOS users: If the extension is hidden in the file name, you can either add the extension manually or go to “Get Info” (item on the File dropdown menu) and unselect “Hide extension.”
Submitting to Turnitin via D2L
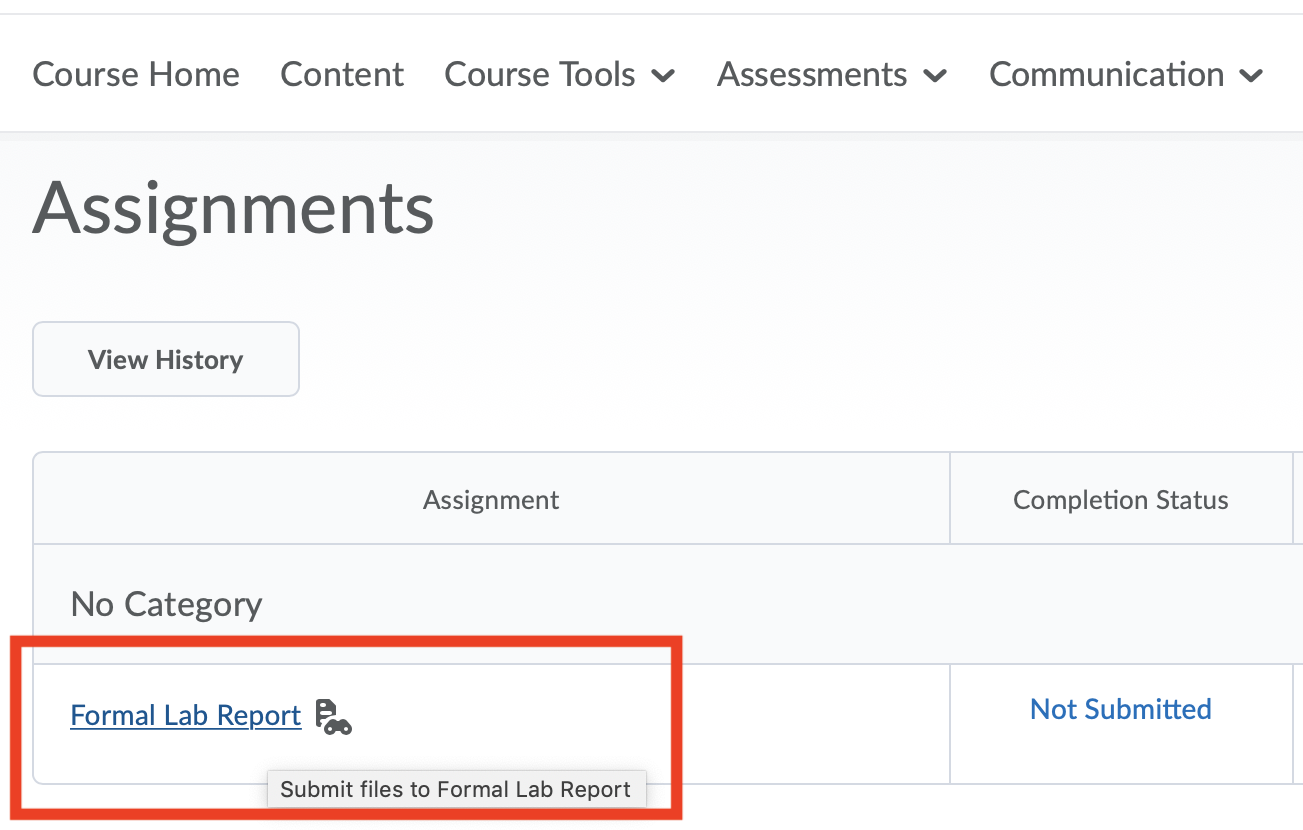
All files are to be turned in on to the course website on D2L. Once you go to the D2L course page, the report can be uploaded by navigating to the Assessments >> Assignments page (see screenshots below).
Note: Be certain you are uploading the correct document! Only the most recent submission will be graded.
If it is a group document submission, everyone will have access to the file uploaded by one team member. Only one team member needs to turn it in.
Follow the instructions on the next page for document submission on D2L:
- Navigate to the Assignments page after logging in to D2L.
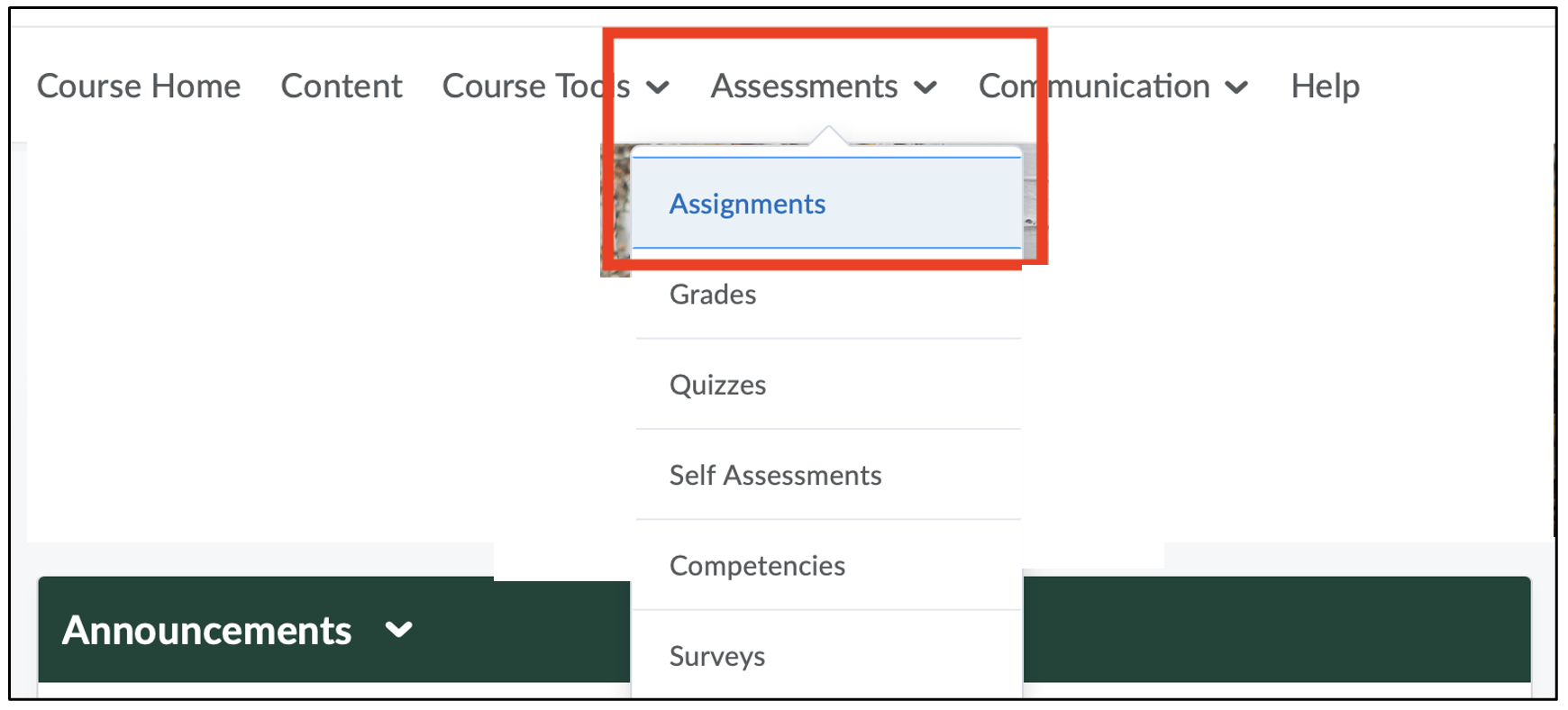
- Navigate to the assignment submission page.
- Upload your assignment and then click “Submit”.
Sample Formal Project Report
An Investigation into the Structure and Properties of an Unknown Acid
Introduction
The goal of this laboratory project was to investigate the structure and properties of an unknown compound which had been found in an unmarked bottle in a local high school laboratory. The team of investigators was called in when a new high school chemistry teacher noticed the bottle sitting on the back of a shelf. The teacher asked the retired teacher who formerly occupied her classroom what it could be, and they narrowed down the possibilities to nine acids: formic acid, acetic acid, citric acid, lactic acid, phenol, salicylic acid, pyruvic acid, 3-nitrobenzoic acid, and 4-nitrobenzoic acid.
Hazardous waste is regulated by the Environmental Protection Agency (EPA) under the Resource Conservation and Recovery Act1. Considering that only one of the possible identities of the unknown acids (citric acid) is considered nonhazardous by EPA and State of Michigan standards2, it was especially critical that the acid was identified in preparation for disposal. Identifying the acid would enable the teacher to safely discard the unwanted chemical.
Experimental
First, the physical appearance and odor of the unknown compound were observed. The melting point was determined using a Mel-Temp apparatus.
Solubility Tests (Qualitative).
A small amount (about 0.100 g) of the unknown was added to 2 mL of the solvent in a test tube and shaken. The test tube was observed to see if any of the solid had dissolved. The solvents used were toluene, acetone, methanol, water, 0.1 M HC1 and 0.1 M NaOH.
Solubility Tests (Quantitative)
Acetone, Methanol, Water, and HCl: The compound was too soluble in acetone and methanol, and not soluble enough in HCl and water, to test the quantitative solubility.
NaOH: The unknown compound (0.205 g) was placed in a small Erlenmeyer flask and 5.00 mL of 0.1 M NaOH was added. The mixture was stirred and heated slightly for about 15 minutes. A watch glass was placed on top of the flask, and the solution was left to cool for one week. The resulting mixture was filtered, and the solid was dried to constant weight.
Chemical Tests
Aliphatic Alcohols: The ceric nitrate test3, which indicates the presence of aliphatic alcohols if a color change occurs upon addition of ceric nitrate, was performed by putting ceric nitrate (5 drops) into a porcelain test plate, to which a small amount of the unknown (dissolved in a few drops of acetone) was added.
Aldehydes and Ketones: The 2,4-dinitrophenylhydrazine (DNP) test4, which indicates the presence of aldehydes or ketones if a precipitate forms upon addition of 2,4-dinitrophenylhydrazine (DNP), was performed by adding a drop of an ethanolic solution of the unknown to a few drops of the DNP reagent in a porcelain test plate.
Aromatic Alcohols: The ferric ion test5, which indicates the presence of aromatic alcohols if a color change occurs upon addition of Fe3+ ion, was performed by adding a drop of an ethanolic solution of the unknown to a few drops of the FeCl3 in a porcelain test plate.
Molecular Weight Determination
A small amount of the unknown acid (about 0.25 g) was dissolved in 200 mL of water upon heating. An acid-base titration was performed with 0.100 M NaOH, using phenolphthalein as an indicator. For thoroughness, the titration procedure was performed three times.
Spectroscopic Analysis
A small amount of the unknown acid on the tip of a spatula added to an NMR tube and dissolved in deuterated acetone in preparation for 1H NMR analysis. The spectrum was acquired on a Varian 300 MHz NMR spectrometer.
Results
The physical properties, including the appearance, odor, and melting point, of the unknown compound are provided in Table 1.
| Appearance of compound | Cream-colored needles |
|---|---|
| Smell | None |
| Melting point | 135.2 - 138.9 °C |
The results of the qualitative solubility tests are provided in Table 2. The color of the resulting solution and the relative solubilities are described.
| Solvent | Color of Solution | Solubility |
|---|---|---|
| toluene | pale yellow | slightly soluble |
| acetone | pale yellow | very soluble |
| methanol | pale yellow | very soluble |
| water | pale yellow | slightly soluble |
| dilute HC1 | pale yellow | insoluble |
| dilute NaOH | deep yellow | very soluble |
The quantitative solubility of the unknown compound in 0.1 M NaOH is provided in Table 3. The calculation to determine the quantitative solubility from the data is shown in Equation 1.
| Starting Mass | Recovered Mass | Dissolved Mass | Volume NaOH | Solubility |
|---|---|---|---|---|
| 0.205 g | 0.095 g | 0.110 g | 5.00 mL | 22 g/L |
[latex]\frac{0.11 \, \text{g}}{5.00 \, \text{mL}} \times \frac{1000 \, \text{mL}}{1 \, \text{L}} = 22 \, \text{g/L}[/latex]
Equation 1. Calculation of quantitative solubility
The results from the qualitative chemical analyses for the presence of various functional groups (aliphatic alcohols, aldehydes or ketones, and aromatic acids), are provided in Table 4.
| Test | Observation | Result |
|---|---|---|
| Aliphatic alcohols | No color change | negative |
| Aldehydes or ketones | No precipitate formation | negative |
| Aromatic alcohols | No color change | negative |
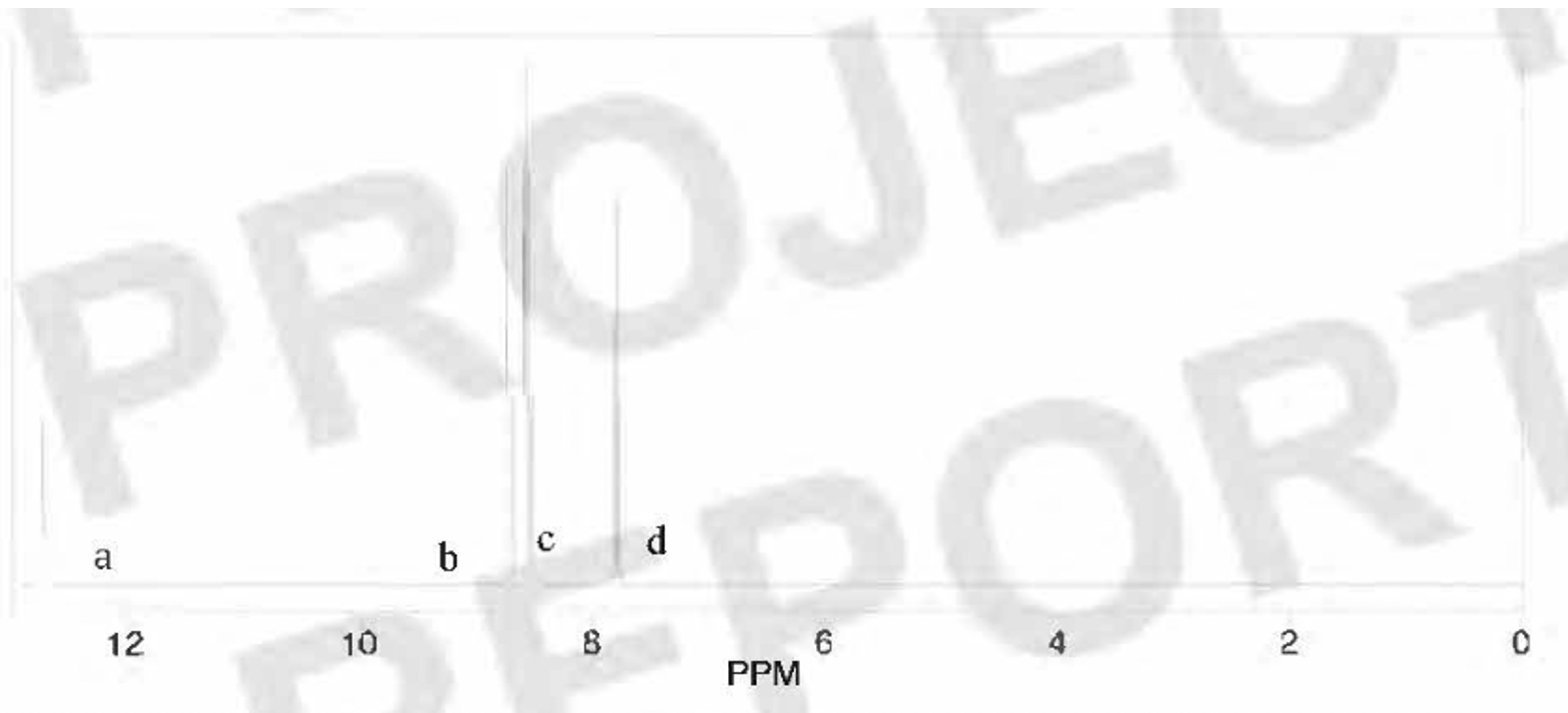
The 1H NMR spectrum of the unknown acid is shown in Figure 1. There were four signals in the spectrum: 12.74 ppm (Ha, 1H, broad singlet), 8.70 ppm (Hb, 1H, singlet), 8.54 ppm (He, 2H, multiplet), and 7.79 ppm (Hd, 1H, triplet).
The data from the titrations, along the resulting molecular weights, are shown in Table 5.
| Trial | Mass of Unknown Acid | Volume of 0.100 M NaOH added | Molecular Weight |
|---|---|---|---|
| 1 | 0.242 g | 14.49 mL | 167 g/mol |
| 2 | 0.251 g | 15.12 mL | 166 g/mol |
| 3 | 0.248 g | 15.00 mL | 165 g/mol |
Assuming a monoprotic acid, the results shown in Table 5 were calculated according to the example below:
[latex]14.49 \, \text{mL NaOH} \times \frac{1 \, \text{L}}{1000 \, \text{mL}} \times \frac{0.100 \, \text{M NaOH}}{1 \, \text{L NaOH}}= 1.449 \times 10^{-3} \, \text{mol acid}[/latex]
Equation 2. Calculation of moles of unknown acid from titration data
[latex]\frac{0.251 \, \text{g}}{1.449 \times 10^{-3} \, \text{mol}} = 167 \, \text{g/mol}[/latex]
Equation 3. Calculation of molecular weight of unknown from titration data
From the three trials, the average molecular weight of the unknown acid was found to be 166 g/mol.
Discussion
Compared to the literature value for the molecular weight of 3-nitrobenzoic acid (167.12 g/mol), the percent error of the experimentally determined molecular weight was 0.6%, as shown in Equation 4:
[latex]\% \text{error} = \frac{167.12 \, \text{g/mol} - 166 \, \text{g/mol}}{167 \, \text{g/mol}} \times 100\% = 0.6\%[/latex]
Equation 4. Percent error for molecular weight of 3-nitrobenzoic acid
The unknown compound was composed of yellow crystals which had the form of long needles under a magnifying glass. The compound was odorless as indicated in Table 1. Solubility tests indicated that the compound was very soluble in both acetone and methanol, and slightly soluble in water and toluene as indicated in Table 2.
This information indicated that the compound was quite polar in nature since it was not very soluble in toluene which is a nonpolar solvent. The compound was not very soluble in water but was soluble in acetone and methanol, leading to the conclusion was that the compound was a polar molecular compound, supporting the suggestion that this was an organic acid.
The compound was insoluble in dilute acid (HCl); however, it was soluble in dilute base (NaOH). Solubility in base implies that the compound must have acidic properties. A determination of the quantitative solubility of the compound showed that the solubility was 22g/L in 1.0 M NaOH. The pH of a slurry of the compound was tested and found to be about pH
At first the compound was thought to be insoluble in water, but some must have gone into solution; otherwise the pH of the solution would have been 7. (The pH of pure water was measured with the same pH meter to make sure the meter was functioning properly).
The tests of physical properties gave some insight into the nature of the compound, but further chemical tests were needed to identify the compound. As indicated in table 4, tests for organic functional groups such as aliphatic alcohols, aldehydes and ketones, and phenols were negative. The negative test for aliphatic alcohols ruled out lactic acid and citric acid. The negative test for aldehydes and ketones ruled out pyruvic acid. The negative test for phenols ruled out phenol and salicylic acid. The remaining possibilities were formic acid, acetic acid, and the two nitrobenzoic acids; thus, further analysis was necessary to determine the identity of the acid.
Since the compound was an acid, a titration could provide more information. The acid was assumed to be a monoprotic acid because all polyprotic acids (e.g., citric acid) had been ruled out. The compound was not very soluble in water, but if 0.25 g acid was heated in about 200 mL of water, it would go into solution (at first, the titration was attempted with methanol as a solvent since the acid is much more soluble in this solvent; however, Sabnis et al.6 indicated that pH titrations are only valid in aqueous solutions). Since all the pH meters were in use, an indicator was used to tell us the endpoint of the titration. An indicator that would change in the pH range of around pH 9-10 was needed, so phenolphthalein was chosen from the chart in the lab manual7. As indicated in Table 5, the three titrations led to an average molecular weight for the unknown of 266 g/mol. This result ruled out formic acid (46 g/mol) and acetic acid (60 g/mol), and it closely matched the molecular weight of nitrobenzoic acid. Because there were two possible nitrobenzoic acids, additional testing was needed to discriminate between them. 3-nitrobenzoic acid and 4-nitrobenzoic acid are isomers; thus, they differ only in the connectivity of the atoms. First, the experimentally determined melting point (135.2 - 138.9 °C) was compared to the literature values for the two remaining possibilities. The melting point of 3-nitrobenzoic acid8 was 139-141 °C, while that of the 4-nitro isomer9 was 237-240 °C. Usually, the presence of impurities will lower and broaden the melting point range. From this information, it appeared that our compound was 3-nitrobenzoic acid with some impurities. To confirm this, a 1H NMR spectrum was acquired. As shown in Figure 1, the NMR spectrum revealed four chemically distinct hydrogen atoms. By comparing the structures of 3-nitrobenzoic acid and 4-nitrobenzoic acid (Figure 2), we saw that 3-nitrobenzoic acid has five types of H atoms, while 3-nitrobenzoic acid has only three. At first this was mystifying because the spectrum seemed not to match either compound; however, upon closer inspection, it appeared that the peak at 8.54 ppm was actually two different peaks overlapping, indicating two distinct types of H atoms. Therefore, the spectrum did indeed total five signals, in agreement with what was expected for 3-nitrobenzoic acid. The multiple peaks around 7.79, 8.54, 8.70 ppm (b, c, d) indicates the four aromatic protons on the benzene ring (Hb, He, Hd). The singlet peak at 12.74 ppm (a) refers to the one proton (Ha) on the carboxylic acid group. The splitting pattern follows "N+1" rule, where N refers to the neighboring protons. Integration refers to the number of protons in this specific chemical environment. Due to the presence of conjugation (benzene ring) and the carbonyl group, all protons were deshielded. This explains the large chemical shift.
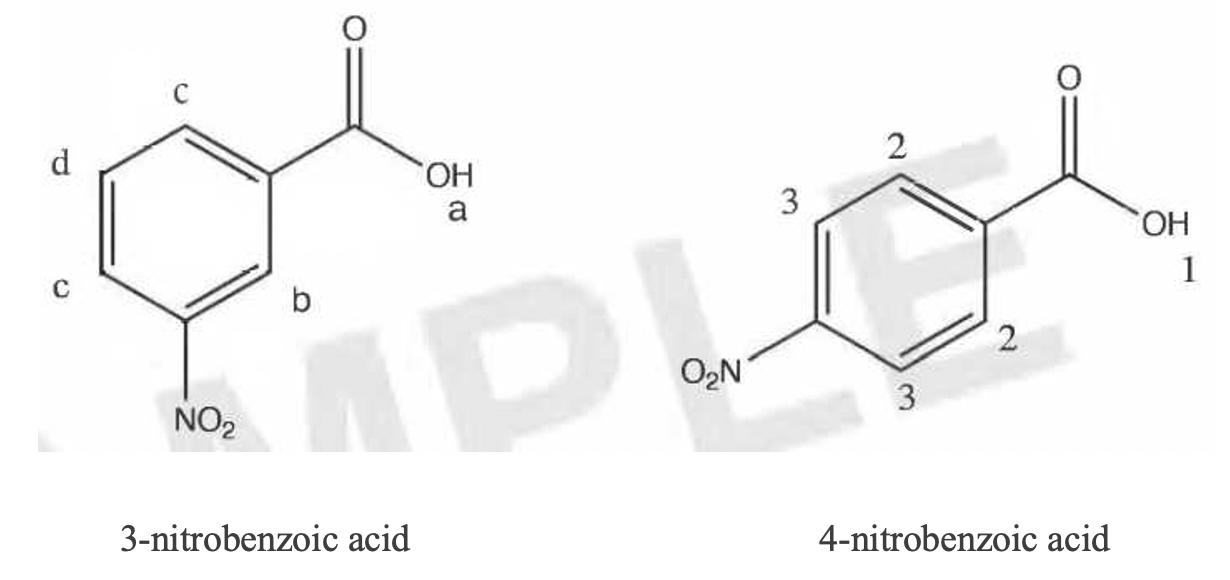
Numbers indicate chemically unique H atom locations.
Altogether, the qualitative tests, titration data, and spectroscopic data showed that the identity of the unknown acid was 3-nitrobenzoic acid. To determine how to dispose of the acid safely, the corresponding SDS8 was retrieved. Using the disposal and toxicity information stated that the compound has an LD50 (oral, rat) of 1950 mg/kg, which indicates that the compound is not very toxic (compared to the sodium cyanide which has an LD50 of 6.4 mg/kg). However, it would be unacceptable to discard it in the trash. Indeed, the SDS states that one seeking to dispose of this compound should “offer surplus and non-recyclable solutions to a licensed disposal company” and “contact a licensed professional waste disposal service to dispose of this material”8. Therefore, the research team recommends that the material be placed in a labeled, sealed container until the local professional waste disposal company can dispose of it as an inert organic compound.
Conclusion
The unknown compound was identified as 3-nitrobenzoic acid by a combination of tests of physical properties, such as melting point and solubility, chemical tests, and spectroscopy. The molecular weight was found by titration to be within 0.6% of the actual molecular weight. Since the SDS sheet indicated that the compound was relatively inert and non-toxic, it is recommended that the compound be disposed of by a professional disposal company, but no other special precautions need to be taken.
References
- EPA Hazardous Waste. https://www.epa.gOv/hw/learn-basics-hazardous-waste#hwid (accessed April 11, 2019).
- Michigan State University Waste Disposal Guide.
https://ehs.msu.edu/_assets/docs/waste/msu-waste-disposal-guide.pdf (accessed April 11, 2019). - Duke, F.R.; Smith, G.F. Ind. Eng. Chem. Anal. Ed., 1940,12, 201—203.
- RSC LeamChemistry.
http://www.rsc.org/leam-chemistry/resource/resQ0000549/bradys- test-for-aldehydes-and-ketones?cmpid=CMP00008335 (accessed April 11, 2019). - CU Boulder Lecture Demonstration Manual General Chemistry.
https://www.colorado.edu/lab/lecture-demo-manual/o638-identification-phenols-ferric- chloride-test (accessed April 11, 2019). - Sabnis, R. W.; Ross, E.; Kdthe, J.; Naumann, R.; Fischer, W.; Mayer, W.; Wieland, G.; Newman, E. J; Wilson, C. M. Indicator Reagents. Ullmann's Encyclopedia of Industrial Chemistry [Online]; John Wiley & Sons, Posted April 15, 2009.
https://dx.doi.org/10.1002/143560Q7.al4_127.pub2 (accessed April 11, 2019). - Cooper, M.M.; Day, E.L.; Duffy, E.M.; Pollock, A.M.; Posey, L.A.; Ward, J.S. Cooperative Chemistry Laboratory Manual. Michigan State University, East Lansing, ML Student laboratory manual, 2019.
- Sigma-Aldrich MSDS - 184329.
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&langu age=en&productNumber=185329&brand=ALDRICH&PageToGoToURL=https%3A%2 F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3Dnitrobenzoic%2Bacid %26interface%3DAll%26N%3D0%26mode%3Dmatch%2 (access April 11,2019). - Sigma-Aldrich MSDS-461091.
https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&langu age=en&productNumber=461091 &brand=ALDRICH&PageToGoToURL=https%3 A%2 F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3Dnitrobenzoic%2Bacid %26interface%3DAll%26N%3D0%26mode%3Dmatch%2520partialmax%261ang%3Den %26region%3DUS%26focus%3Dproduct (accessed April 11, 2019).
This chapter was adapted from Erin Duffy's work in Cooper, M. M. et al Cooperative Chemistry for Michigan State University, 2019.

