Part 5: Organic Laboratory Techniques
19 Filtration
Filtration is a technique used for separation of mixtures of solids and liquids. The type of filtration apparatus used depends upon whether you want the solid you are filtering or the liquid (called the “filtrate”) from the mixture.
- If the desired compound is the filtrate, a simple gravity filtration is the best method.
- If the desired compound is the solid, a vacuum filtration may recover more solid.
Find a video tutorial at Filtration | MIT Digital Lab Techniques Manual (Gravity filtration begins at 5:06).
Gravity Filtration
In gravity filtration, a conical funnel is fitted with a piece of filter paper that has been folded. In simple fold, shown below on the left, the filter paper is folded in half to form a semicircle. The folded edge is then folded again to form a quarter circle. “Fluted fold”, shown below on the right, is also used for rapid filtration with maximized exposed surface area of the paper.
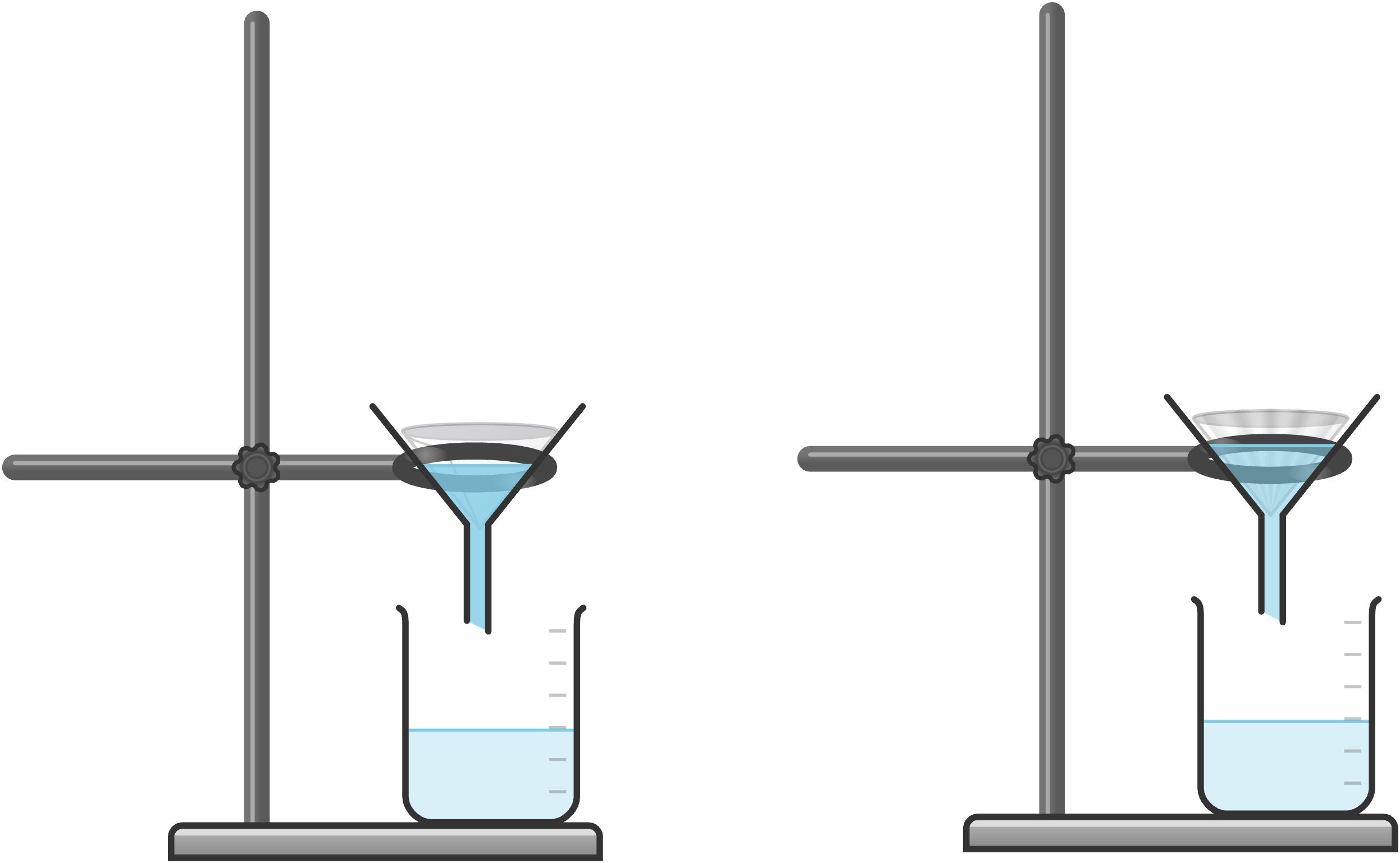
Wet the filter paper with the solvent you are using; for example, if the mixture is a solid and water, use water to wet the paper inside of the funnel. Pour the mixture of solid and liquid through the filter paper and funnel and allow it to filter by gravity. The solid remains as the liquid passes through the filter paper. Keep adding the mixture to the funnel as the level drops. You may find it necessary to swirl the mixture as you pour so that the solid does not get left behind in the bottom of the flask.
There are different grades of filter paper, so if the solid is finely divided, you may have to use a different grade of filter paper. Using the wrong grade of filter paper may result in poor or no separation, which may be visible from the appearance or testing of the filtrate. In general, the finer the filter paper, the slower the filtration.
When you have filtered all the material, you may find that there is some solid left in the original flask. Pour some of the filtrate back into the original flask with the remaining solid, swirl, and re-filter. Repeat this process until you have all the solid in the filter funnel.
Then, wash the solid by pouring a small amount (<5 mL) of fresh cold solvent through the funnel. Repeat the washing. (Note: for washing solids, two small portions are more effective than one large portion.) The filtrate is now ready for further use. If you also need the solid, you should allow it to dry on the filter paper before removing it to be weighed.
Vacuum Filtration
This type of filtration is usually performed when it is primarily the solid that is the desired compound. Take a Buchner funnel (or Hirsch funnel) and a Buchner flask (the “vacuum filtration” flask that has a glass side arm) and attach the thick-walled rubber hoses to the side arm of the flask as well as the aspirator or vacuum.
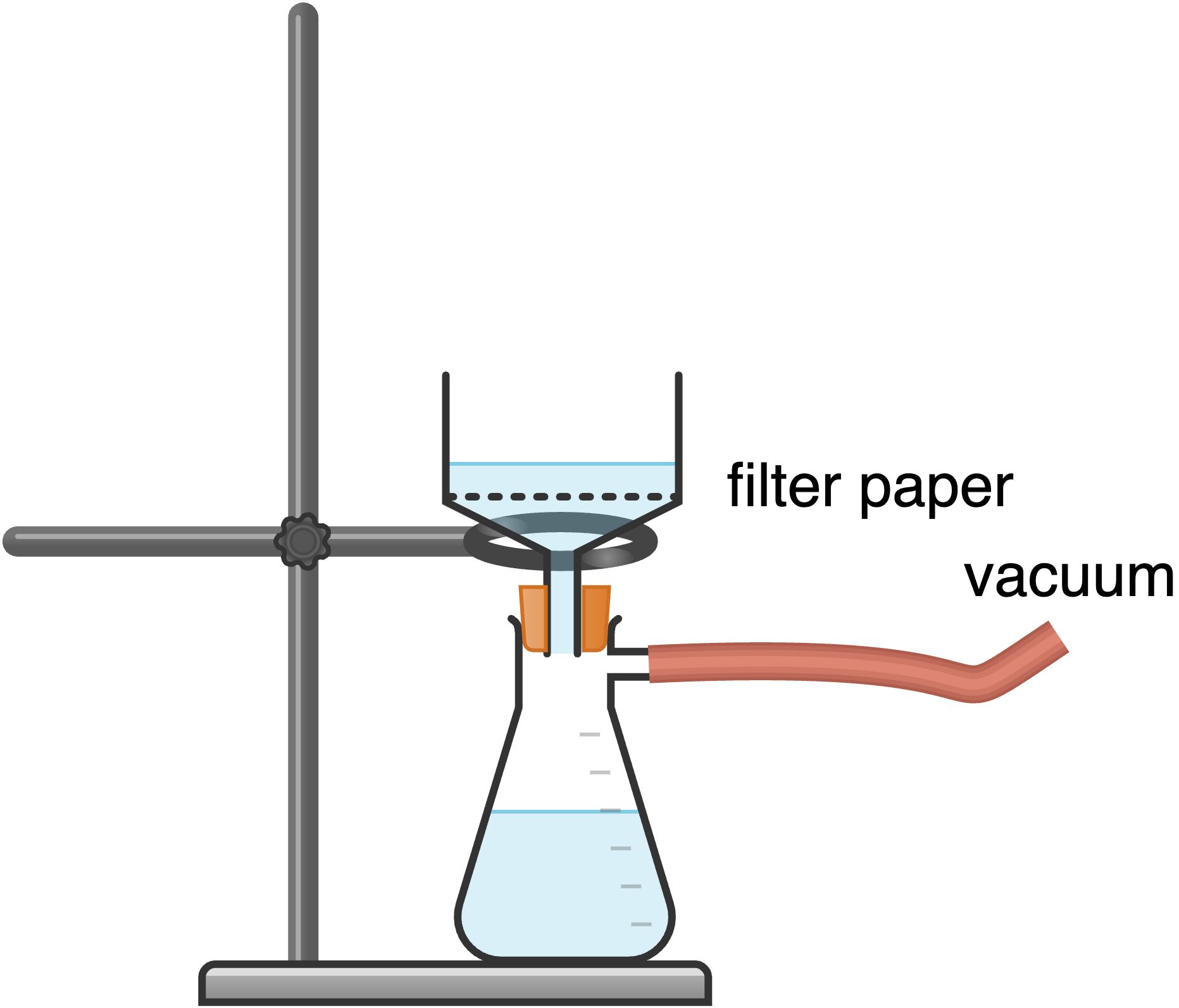
Once the flask is secured to a ring stand and attached to the aspirator via the rubber hose, place the funnel (along with the neoprene adaptor) into the neck of the flask. Place an appropriately sized, pre-weighed piece of filter paper over the holes in the Buchner funnel; the filter paper in this method should not be folded and should fit the size of the funnel such that all holes are covered. Wet the filter paper with the solvent you are using and turn the aspirator on full (partially turning on the water could cause water from the aspirator to get sucked into the Buchner flask).
While occasionally swirling the vessel with your mixture, slowly pour into the funnel, aiming for the center of the filter paper, and allow the mixture to be filtered by the action of the vacuum. Wash out any solid that remains in the original flask using filtrate, as described in the previous section. When all the material has been filtered, wash the solid (sometimes referred to as the “filter cake”) as previously described. Allow the solid to dry on the filter paper, and then find its mass.
Note: Before turning off the aspirator, disconnect the vacuum hose. Otherwise, water will suck back into your flask.
The majority of this chapter has been adapted from the CEM 161/162 manual: Cooper, M. M. et. al. Cooperative Chemistry for Michigan State General Chemistry Laboratories, 2019.

