Part 5: Organic Laboratory Techniques
18 Extraction and Drying
There are two different extractions: solid-liquid extraction and liquid-liquid extractions. Both techniques use solubility differences to transfer the target compound from one phase to another.
Solid-Liquid Extraction
Solid-liquid extraction is a method used to separate a compound or compounds from a solid matrix using a solvent. The solute is extracted from solid phase to the carrier liquid phase due to good solubility. The solid impurities residue is then removed by filtration. Carrier liquid will be removed by rotary evaporation (rotovap) or distillation.
Solid-liquid extraction is commonly used in various industries, including pharmaceuticals, food processing, and environmental analysis, to extract and purify compounds from solid materials.
Liquid-Liquid Extraction
Liquid-liquid extraction is a method used to separate compounds based on their relative solubilities in two immiscible liquids. Generally, you will be doing an extraction to separate the organic product (in an organic solvent such as ether or ethyl acetate) from an aqueous layer. Depending on your product, you may want to retain either the aqueous layer or the organic layer. Use density to navigate which layer is which.
Large Scale
To separate volumes larger than a few milliliters, you will need a separatory funnel. This piece of equipment consists of a pear-shaped container with a stopcock at one end and a ground-glass opening at the other.
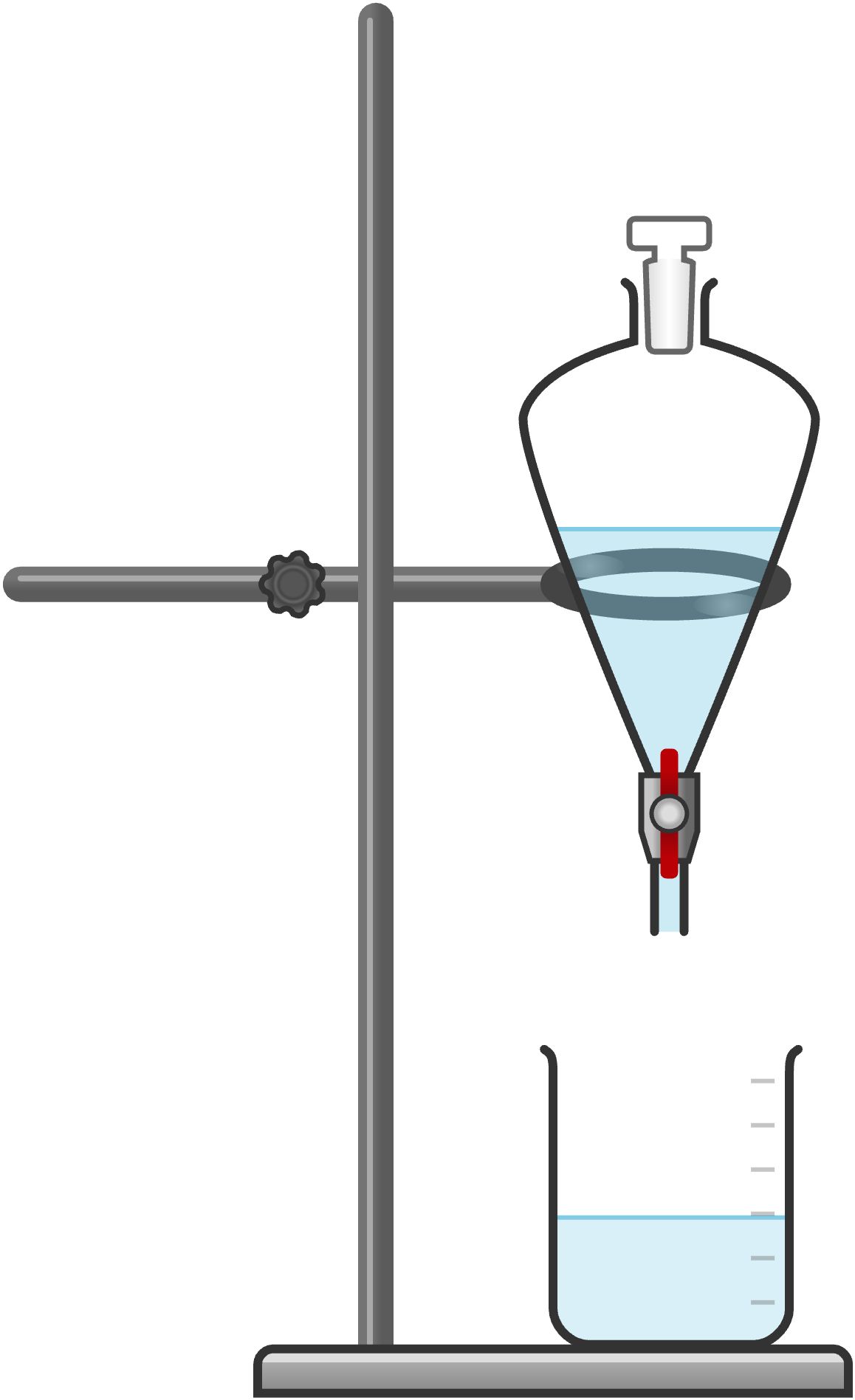
To use the separatory funnel (often referred to as just “sep” funnel) to perform an extraction or separation, place the two immiscible liquids you wish to separate in the sep funnel. Put the ground-glass stopper in the open end, and ensure that the stopcock is closed (the knob is horizontal, i.e., parallel to the floor); vigorously shake the funnel while firmly holding the glass stopper in place. Pressure will build up quickly if the solvent is volatile. Making sure to point the sep funnel away from yourself and anyone else. Vent the funnel by pointing the tip up and away from you and opening the stopcock. You should hear a “whoosh” or a release of pressure. Close the stopcock and repeat this process several times. The funnel can now be placed in an iron ring on a ring stand. Remove the ground glass stopper before you open the stopcock. If you forget and open the stopcock while the stopper is still in the funnel, the fluid will be trapped in the sep funnel because you have formed a vacuum. If this happens, close the stopcock and remove the ground-glass stopper. Now open the stopcock and allow the bottom layer to drain out into an appropriate container. Slow the rate of flow of the fluid as the interface of the two layers approaches the stopcock. At this point, you might achieve best results by draining the bottom layer dropwise. Close the stopcock when the last drop of the bottom layer is in the stopcock. You can then pour the top layer out of the open end of the sep funnel, so it will not be contaminated by the lower layer.
Usually, it will be obvious which layer is the organic layer and which is the aqueous layer. If you get confused, try adding a drop or two of water to one layer and see which layer the added water joins. In general, if the organic layer is in ether or ethyl acetate, it will be the upper layer (and the aqueous will be the lower layer). If the organic solvent is denser, such as dichloromethane or chloroform, the organic layer will be the bottom layer.
Small Scale
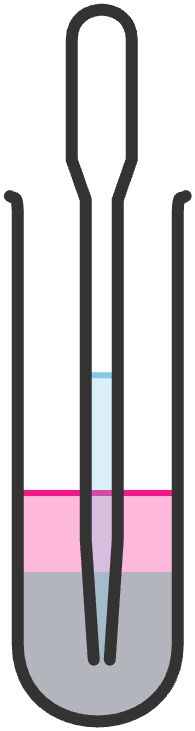
If the volume of solvent that you want to extract is only a few milliliters, the sep funnel is too big to be efficient. In this case, you should use microscale extraction vials.
Place the mixture that you are extracting into a conical vial; cap and shake to assure thorough mixing. Uncap the vial and let it stand to allow sufficient separation of the layers.
If the layer you are extracting is on the top, draw the lower layer into a Pasteur pipette; the conical nature of the vial will allow you to make a good separation. Pipette the layer you have drawn into another container.
Alternatively, if the layer you are extracting is at the bottom, take all of the mixture up into the Pasteur pipette and gently tap the pipette to make sure the layers are well separated within the pipette. Then pipette the bottom layer (the desired layer in this case) into one clean vial and the top layer into another vial. Add more ether to the bottom layer and repeat at least twice more, combining the organic extracts into the other vial.
Drying Organic Extracts
When you have extracted an aqueous solution with an organic solvent, you need to remove any water that has dissolved in the organic layer or that may have been accidentally included into the ether extracts.
The easiest way to dry an organic solvent is to treat it with an anhydrous inorganic salt, such as magnesium sulfate, sodium sulfate, calcium chloride, etc. These salts must be anhydrous or else the water in the organic layer will not be absorbed.
The amount of drying agent needed depends on the amount of water residue in the organic mixture. For example, for 50 mL of solvent, try a couple spatulas of drying agent first. If the drying agent seems wet and clumpy, add some more until all the moisture is absorbed and some of the drying agent is freely moving. Allow it to sit for a few minutes and then filter off the drying agent.
Note: The majority of this chapter has been adapted from the CEM 161/162 manual: Cooper, M. M. et. al. Cooperative Chemistry for Michigan State General Chemistry Laboratories, 2019.

