Appendix
36 Synthesis and Analysis of Painkillers–Planning Scenario and Background
Scenario
Your team has been employed by a drug development division of a start-up company that has decided to enter the arena of manufacturing over the counter (OTC) painkillers (also known as analgesics). Common ingredients for analgesics may include:
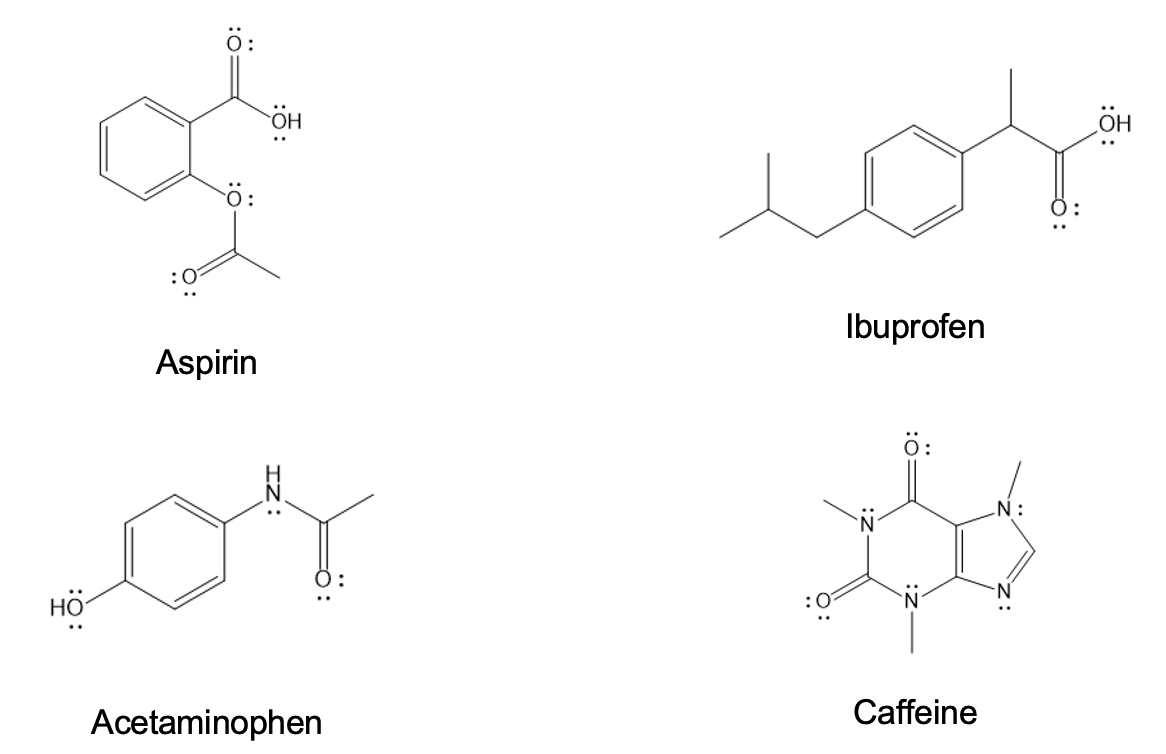
The first and most commonly produced pain reliever was aspirin, and it is frequently featured in various formulations of analgesics. Although it is quite easy to synthesize, pharma start-ups like the one you’re employed at must balance the demand from consumers and hospitals, regulatory pressures, and profit margins to justify their investment.
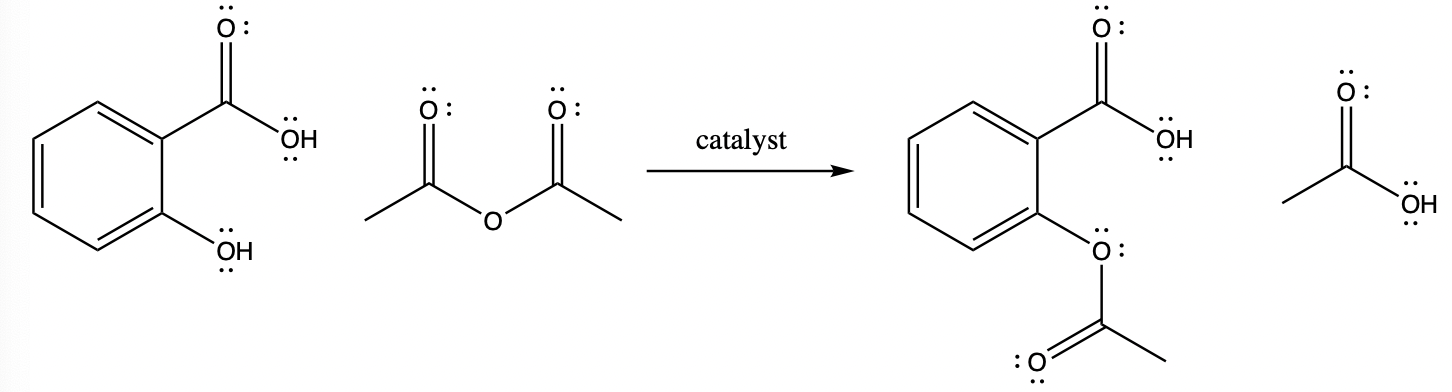
The start-up company has tasked your team with looking for a low-cost green alternative to satisfy EPA requirements and shareholders. Your team is going to explore ways to synthesize aspirin (as shown below) in order to achieve the highest yield, the greenest synthesis, and the most economical synthesis.
After your team has optimized the synthesis of aspirin to satisfy the conditions above, your second task is a consultation with Ingham County Health Department. Patients have been reporting to the emergency rooms in the greater mid-Michigan area after taking a generic OTC analgesic. Many analgesics have more than one active ingredient (or component). Each team member will be given a sample of the generic analgesic and asked to analyze it. You will need to report what the active ingredients are in the binary-mixture sample (part 3). These results will be used to trace the production issues.
Tasks for successfully completing this project
- Determine which catalyst is greenest and most economical to synthesize aspirin in high yield and provide confirmation of the purity.
- Use three identification techniques to prove the isolated product from the synthesis is the intended product.
- Compare the four different routes from a green chemistry perspective: efficiency, safety, waste prevention, cost, and greenness.
- Analyze the sample of generic OTC analgesic using thin layer chromatography (TLC) to identify the active ingredients in the binary mixture.
Special materials available for this project
In addition to the necessary reagents, salicylic acid and acetic anhydride, your team will test the following catalysts:
Catalysts
| Phosphoric acid | Sulfuric acid | Sodium acetate | γ-valerolactone |
For your consultation with the Ingham County Health Department, a preliminary screening has indicated that the following components are the most likely active ingredients in the generic:
Potential Components in the Generic OTC Analgesic
| Aspirin | Acetaminophen | Acetanilide | Caffeine |
Safety notes:
● Be sure to consult the MSDS/SDS for any compound that you work with.
● Wear safety goggles, gloves, and appropriate clothing at all times in the laboratory.
● Dispose of waste in the labeled containers. Do not pour any waste down the drain unless you check with your instructor first.
● Use great care when transferring solutions of acids and bases.
● If you spill a strong acid or base on your clothes or skin, rinse with large amounts of water immediately and ask one of your team members to tell your TA.
● Salicylic acid is toxic and an irritant. Avoid contact with skin, eyes, and clothing. Avoid breathing the dust.
● Acetic anhydride is toxic, corrosive, and a lachrymator (causes tears). Wear gloves and use in the hood. Avoid contact.
● Sulfuric acid is corrosive and causes burns. Avoid contact.
● The aqueous filtrate from the crystallization step can be diluted with water and poured down the sink.
Techniques you may need to learn or review
Consider reviewing the following sections in the Lab Manual:
- Purification via recrystallization
- Isolation of crude product via vacuum filtration
- Determination of purity
- Separation of components via Thin Layer Chromatography (TLC)
- Identification techniques
- Spectroscopy
Concepts you may need to learn or review
The following sections from the OCLUE (CEM 251/252) textbook may be helpful:
- Chapter 7: Nucleophilic Attack at the Carbonyl Carbon, especially the Esterification section (pages 135-136).
- Chapter 2: Spectroscopy, especially IR (pages 34-39) and 1H-NMR (pages 46-49)
Additional Background
Your literature search has yielded this general procedure for the esterification reaction.
Esterification
- Catalyst: Phosphoric acid, sulfuric acid, or γ-valerolactone
Place 1.0 g salicylic acid and a stir bar in a small beaker, add 2 mL of acetic anhydride (with care in the hood) carefully while stirring the mixture. Add 5 drops of your chosen catalyst. After about 10 minutes place the beaker in a hot water bath so that any remaining solid dissolves and the reaction goes to completion. Pour the resulting solution onto about 20 mL of ice water, wait for crystallization, vacuum filter and dry.
- Catalyst: sodium acetate
Add 1g salicylic acid, 1.00 mL acetic anhydride, 0.3 g sodium acetate and a stir bar into an Erlenmeyer flask or a beaker (with watch glass on top). Stir the reaction at 55 °C for 50 minutes. Add 20 mL ice water into the reaction mixture. Perform vacuum filtration and dry the solid.
Note: Do not copy and paste the procedures above directly onto your notebook. Use your own words (or drawing).
Using Green Metrics and Tools
In your course D2L page, there are links to Green Metrics and Tools. Use appropriate green chemistry metrics or principles to compare the efficiency, safety hazard, waste prevention, costs, and impact of different synthetic routes.
Project Checklist
Use these questions (1) as a guide to determine if you have completed all of the required tasks and (2) to help you to be able to develop a good report.
Part 1: Synthesis of aspirin
- Draw the structures of the catalysts and label the functional groups. For each catalyst, is this molecule acidic, basic, or neutral?
- In your group, design an experimental protocol that will allow you to investigate which catalyst for the aspirin synthesis that (1) will produce the highest yield, (2) is the most economical (price reference is provided as a document), and (3) provides the greenest synthesis. Hint: refer to SDS information of all catalysts used.
- There are many ways to monitor the progress or completion. Propose a theoretical method.
- o Esterification reaction is exothermic. Student L plan to use a rise in temperature to monitor reaction progress. The rate of temperature change can be used to determine the fastest reaction. Would this work? Why or why not?
- How will you control the temperature (55 °C) for the esterification reaction using sodium acetate as catalyst?
- How will you prepare ice water for recrystallization? Remember: the water you added in your solution must be DI water. Ice from ice machine is tap water.
- How will you isolate your product after the reaction? How can addition of ice water assist with precipitation/crystallization? Will the aspirin product be a solid or liquid?
- Which green metrics will you evaluate the various catalysts on? What information will you need to record in order to calculate these?
Part 2: Recrystallization and purity testing of aspirin
- Draw the structure of aspirin. Label out the polar bonds.
- What solvent (solvent mixture) do you think it might be soluble in? Consult the green solvent guide in the lab manual to make your selections.
- According to your online literature research, which solvent is most suitable for recrystallization of aspirin?
- Describe three methods that you will use to prove that the product you isolated from the reaction is indeed the product you expected and not, for example, starting material.
- What data do you need to collect for the following green chemistry comparison? Note that you will need to use at least 4 green chemistry metrics/principles to evaluate the fourth synthesis.
- Calculate the percent yield and atom economy for each reaction. What data do you need to collect for calculation of limiting reagent and percent yield? How will atom economy change using different catalysts?
- Perform an economic analysis using the Cost Analysis Tool and the EcoScale rubric on each method. (Cost of each reagent was in a file on D2L Project 2 folder.)
- Which green metrics will you evaluate the waste created in each route? How would you estimate the waste created in each route?
- What recommendation would you make to the start-up company and justify that recommendation using the three metrics.
Part 3: Analysis of multicomponent analgesics
You will be given a sample which is a binary mixture of the analgesic from the Ingham County Health Department. You also have the pure standards of the potential components from the scenario (acetaminophen, caffeine, acetanilide) and aspirin product that was purified in week 2. Perform a thin-layer chromatography (TLC) experiment to identify the unknown binary mixture.
- Thin Layer Chromatography (TLC) techniques is widely used in organic synthesis for monitoring reaction completion and purity test. How does it work?
- What is Rf value and how can you calculate it?
- Using knowledge in polarity, which of the four standard reagents will have the highest Rf value and the lowest Rf value?
- Using the standards of the pure analgesics provided, how will you identify what your mixture contains?
Chemicals (add rows as needed)
| Chemical Name | Hazard(s) | Accidental Release Measures |
Equipment
Generate a detailed list of equipment you need, hand-draw the apparatus set-up for aspirin synthesis and TLC.
Project Plan (Procedures)
Write a preliminary plan for your experimental procedure for each week. Indicate what each person in your group will do to solve the problem, and what data they will record. Break down the work so you use your time effectively and that each person will end up with a mini lab report for their data and notes assignment next week. Be detailed.

