9 Membranes and Compartments
Learning Objectives
- Use the structure and properties of membrane lipids to develop a model of how and why membranes form and use this model to explain
- how and why changes in membrane components and/or temperature change membrane fluidity
- how and why some types of molecules disrupt membranes
- Describe the functions of key cellular compartments and explain why compartmentalization is advantageous for eukaryotic cells
- Compare and contrast prokaryotic and eukaryotic cells
It’s only 5 nanometers thick (1/200,000th of a millimeter), can assemble itself as well as heal itself, and is responsible keeping the contents of the cell inside the cell. What is being described? It’s a cell membrane! Membranes are universal features of cells—every cell has a plasma membrane that defines the boundary of the cell and forms a barrier to separate the internal components from the external environment. As we will see in later chapters, membranes allow cells to regulate what enters and exits the cell and play a crucial role in processes for harvesting energy and matter in metabolism.
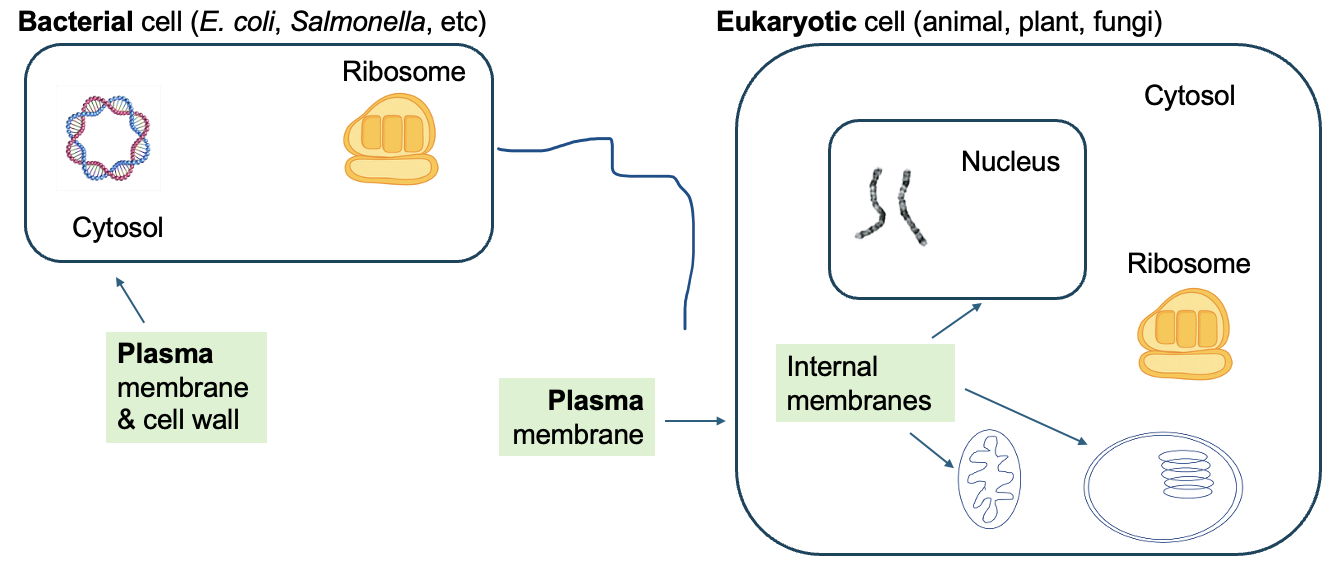
Importantly, the presence of specific membranes in cells is also used to define big groups of life. As shown in Figure 9.1, bacterial cells only have a plasma membrane, meaning that other cellular components are housed within that one internal compartment. In contrast, eukaryotic cells (and our cells are eukaryotic animal cells) have many internal compartments. These compartments are organelles (“little organs”), and each has specialized functions within the cell. One such organelle is the nucleus, a defining feature of eukaryotic cells, which is created by the nuclear membrane or nuclear envelope within the cell. As discussed in earlier chapters, the nucleus is where the genomic DNA is located and where transcription occurs in eukaryotic cells. We’ll discuss the functions of other compartments in later parts of this chapter.
Chapter Outline
Section 9.1 Membrane Components
Section 9.3 Contrasting Bacterial and Eukaryotic Cell Structure
Section 9.4 Eukaryotic Cell Compartments
Section 9.1 Membrane Components
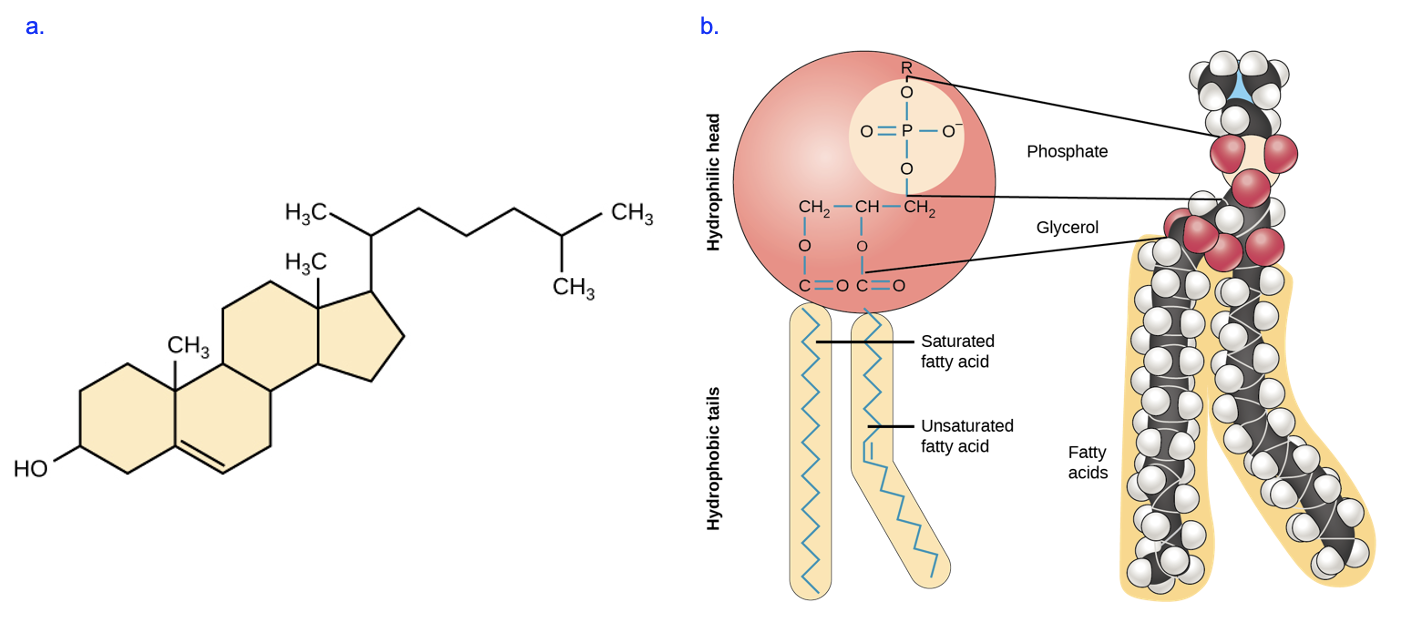
What is a membrane made of? In Chapter 3, we learned that phospholipids and cholesterol (in animal cells) are key components of membranes (Figure 9.2).
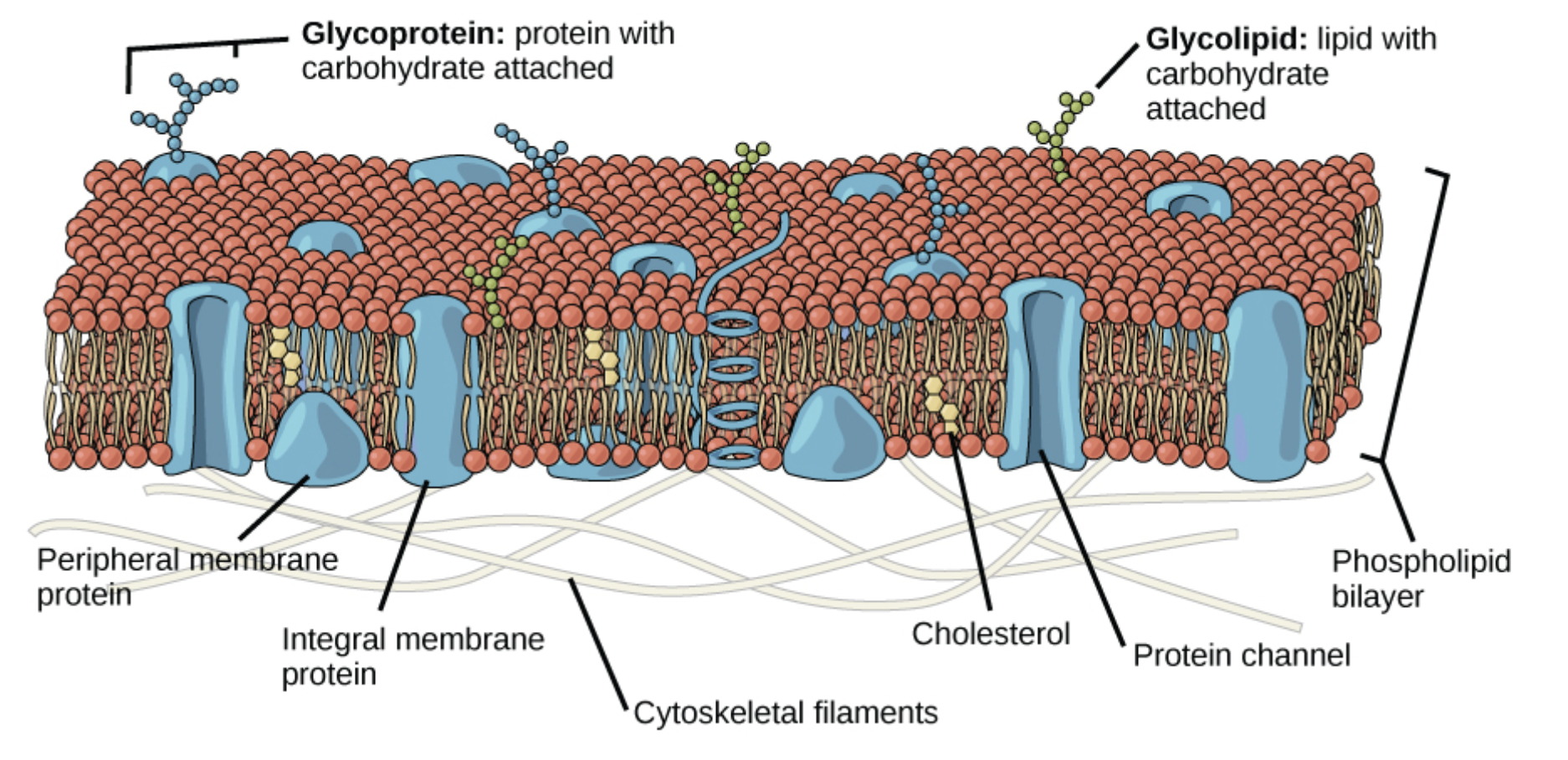
But about 50% of the mass of a membrane is proteins with diverse functions. Figure 9.3 shows a lipid bilayer with many different types of proteins that are typically found in plasma membranes. As we will see in later chapters, transporters, receptors, and enzymes are all types of proteins found in membranes.
Membrane lipids and proteins have a key structural feature in common: they are amphipathic, meaning they contain distinct polar and nonpolar regions. As labeled in Figure 9.2b, a phospholipid has a hydrophilic head group and two hydrophobic fatty acid tails. Try to identify the polar and nonpolar regions in the model of cholesterol (Figure 9.2a).
Figure 9.4 shows the bilayer structure of the membrane. Notice that in this formation, some polar phospholipid heads face the exterior solution, which is aqueous, while others face the interior cytosol of the cell, which is also aqueous. This leaves the fatty acid tails of phospholipids to interact with other the fatty acid tails in the membrane and not exposed to water.
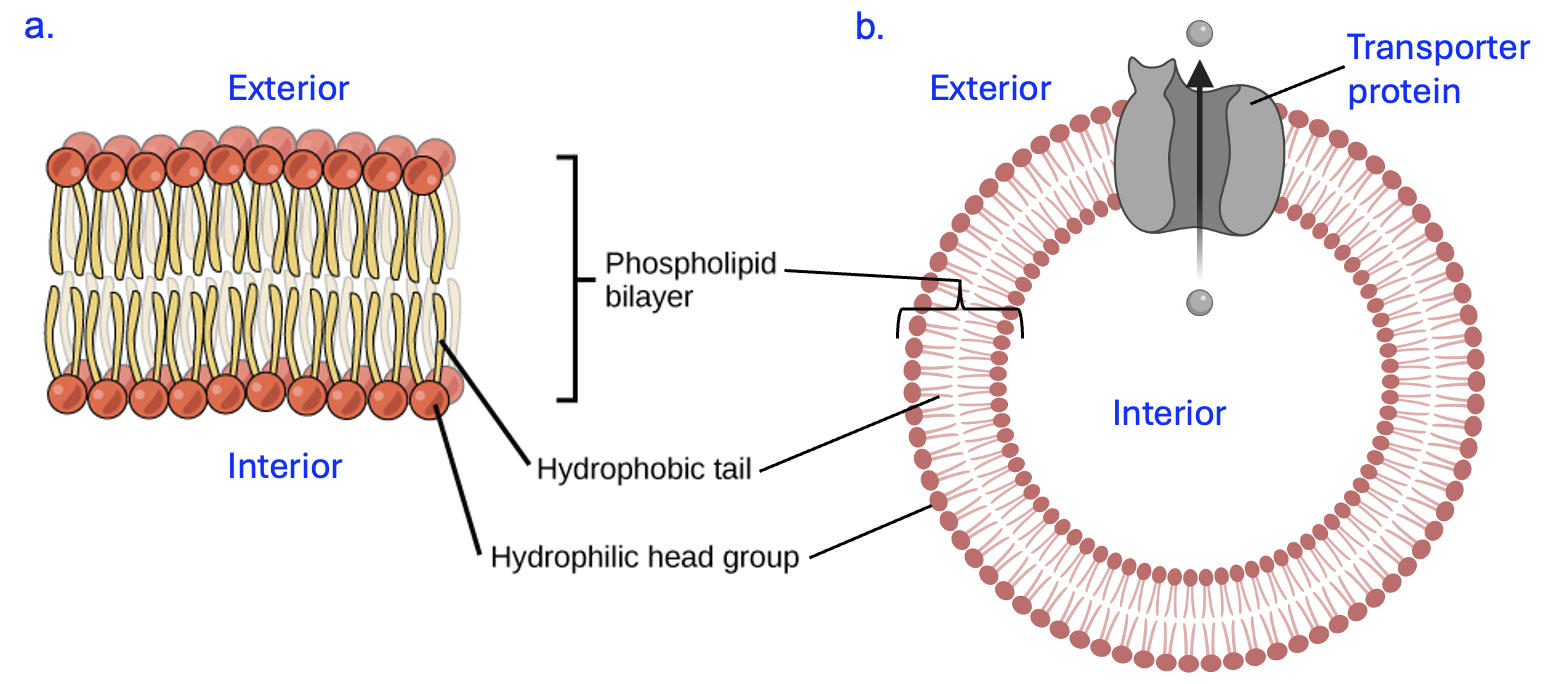
In an aqueous environment at neutral pH, this bilayer forms spontaneously (no energy input is required) due to hydrophobic clustering. Once the membrane forms, London dispersion forces (LDFs) between fatty acid tails can stabilize the structure and affect its fluidity.
Any proteins embedded in the membrane are also amphipathic. Part of the protein must be hydrophobic to interact with the fatty acid tails to remain in the membrane. Other parts of the protein need to be hydrophilic to interact with the aqueous fluids inside and outside of the cell. The protein in Figure 9.4b is a type of transporter protein that we will discuss in more detail in a later chapter. A transporter creates a hydrophilic pore to allows specific molecules to cross membranes. Based on these descriptions, consider what amino acids you would expect to find at each location of this transporter protein. You may wish to review the amino acid structures and return to the protein simulation in Chapter 6 to explore ideas relating to protein folding. What environment (oil versus water) do you think would best mimic the environment found in the core of the lipid bilayer,associated with the fatty acid tails, and why?
How Does Soap Work?
Compare the structure of phospholipids (Figure 9.2b) with the molecule in Figure 9.5a. We can see that this molecule has a charged group interacting with Na+, as well as long hydrocarbon tail that is hydrophobic. The presence of distinct polar and nonpolar regions means this molecule is also amphipathic.
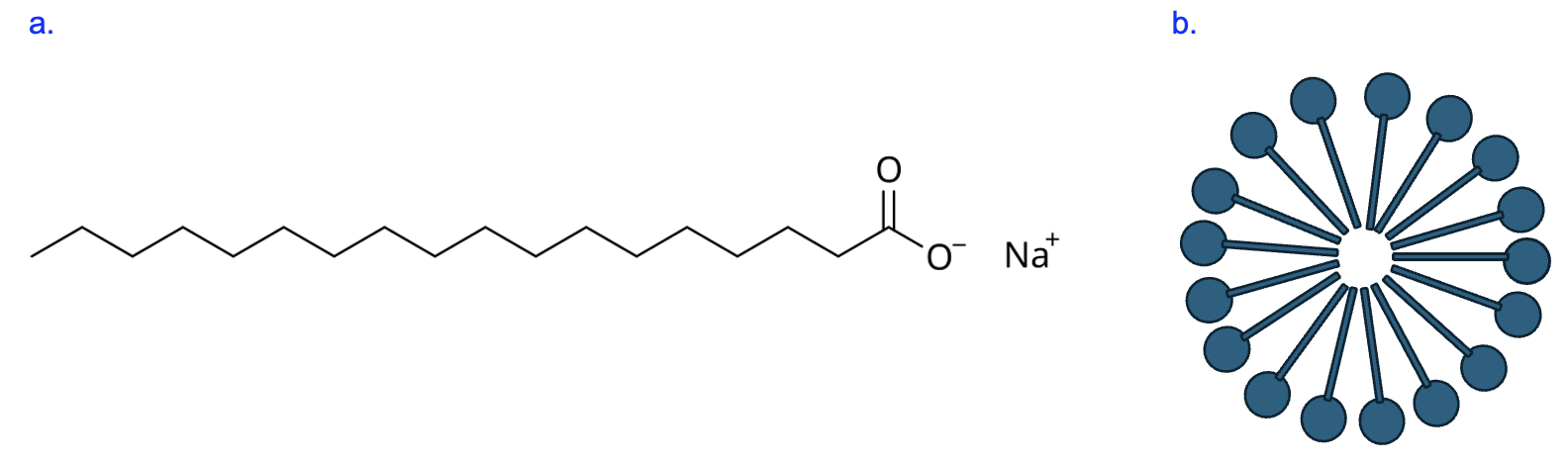
If you had many of these molecules in water, how would you expect these molecules to arrange themselves? Well, just like with phospholipids, the polar head group would interact well with water, while the nonpolar hydrocarbon tail would avoid interacting with water. These tails point towards each other while the head groups remain on the surface, forming a spherical structure called a micelle (Figure 9.5b).
As you may have guessed from the title of this section, the molecule in Figure 9.5a is soap, and we know that soaps are particularly effective and important for cleaning. Their structure helps explain why. Oily substances like grease do not interact with water and are not soluble in water, which is why water alone cannot rinse them away. But soap molecules interact with both water and grease. Soap molecules form micelles around grease particles, with the grease in the center, which are then washed away by running water.
Following similar principles, soap molecules can also dissolve membranes of infectious materials such as viruses. This is a key reason why handwashing is so effective and important for protecting against the spread of disease. For additional explanation of this, watch this video:
Section 9.2 Membrane Fluidity
You can think of membrane fluidity of how easily molecules in the membrane can move—high fluidity means lots of movement. To be an effective barrier and carry out its other functions correctly, membrane fluidity needs to be “just right”—not too loose nor too stiff. As discussed in Chapter 3, the number of LDFs between those fatty acid tails is inversely correlated with the fluidity of the membrane. If more LDFs are present between fatty acid tails, lipid movement slows and the membrane is less fluid (more stiff), while fewer LDFs between fatty acid tails allows more lipid movement and the membrane is more fluid (more flexible).
As shown in Figure 9.2, carbon chains with only single bonds between carbons are saturated (“saturated with hydrogens”), while the presence of at least one double bond between carbons in the chain makes the fatty acid unsaturated. Importantly, the double bond creates a “kink” in the chain that reduces the ability of these fatty acids to pack closely together and interact with LDFs (remember from Chapter 2 that LDFs are extremely weak, so molecules must be very close together for LDFs to have an influence).
Figure 9.6 shows five phospholipids that have either all saturated fatty acids (Figure 9.6a) or have at mixture of saturated and unsaturated fatty acid tails (Figure 9.6b). Visually, we can see that the presence of unsaturated fatty acid tails means the phospholipids cannot be closely packed together, and corresponds to a membrane that is more fluid. Thus, the structure of lipids within the membrane strongly influences the fluidity of the membrane, and having more unsaturated fatty acid tails results in a membrane that is more fluid.

The length of fatty acid tails can also influence membrane fluidity. Compare the lipids in Figure 9.6c or 9.6d with those in Figure 9.6a. Which membrane is most fluid, and which is least fluid, and why? Once again, the number of LDFs is key—a structure that allows for more LDFs (Figure 9.6d) is less fluid than a structure with fewer LDFs (Figure 9.6c). Building fatty acid tails that are shorter is a way to increase membrane fluidity.
Finally, temperature also influences fluidity. If you’ve ever melted butter in a frying pan, you have some personal knowledge of this! Butter that is solid at room temperature turns into liquid as heat is applied. Remember, it’s the LDFs between fatty acid tails that make butter solid at lower temperatures, but a small input of heat energy easily disrupts those interactions. A similar effect is observed on cell membranes—as heat increases, membranes become more fluid. Imagine the cell of a plant growing outdoors. As night turns to day, the increase in air temperature will increasing membrane fluidity. As day turns to night, the opposite effect is observed. Remember that cells require an optimal membrane fluidity for their function. The changes in temperature described above alter the fluidity from that optimal level.
Since most cells cannot change their environment, cells survive by counteracting the changes in fluidity using features they can control, such as the saturation and length of the fatty acid tails in their membrane lipids. If increased temperatures make the membrane too fluid, a cell can alter its lipids to reduce fluidity and return to the optimum. Membranes with fatty acids that are more saturated, or fatty acids that are longer, have decreased fluidity. So, a cell could synthesize more fatty acids of these types and add them to the plasma membrane in response to increased temperatures. To counteract the effects of decreased temperature, cells could synthesize and incorporate phospholipids with fatty acid tails that are more unsaturated or shorter in length.
Section 9.3 Contrasting Bacterial and Eukaryotic Cell Structure
Figure 9.7 shows more detailed representations of a generic bacterial cell (Figure 9.7a) and two types of eukaryotic cells, a generic animal cell (Figure 9.7b) and a generic plant cell (Figure 9.7c). As you can see by comparing structures, and as noted in the introduction, eukaryotic cells can be distinguished from bacterial cells by the presence of many compartments that are created by internal membranes. Bacterial cells, in contrast, just have the plasma membrane to separate the cell contents from the external environment. There is no nucleus to house the DNA, and no internal compartments where specialized functions can occur.
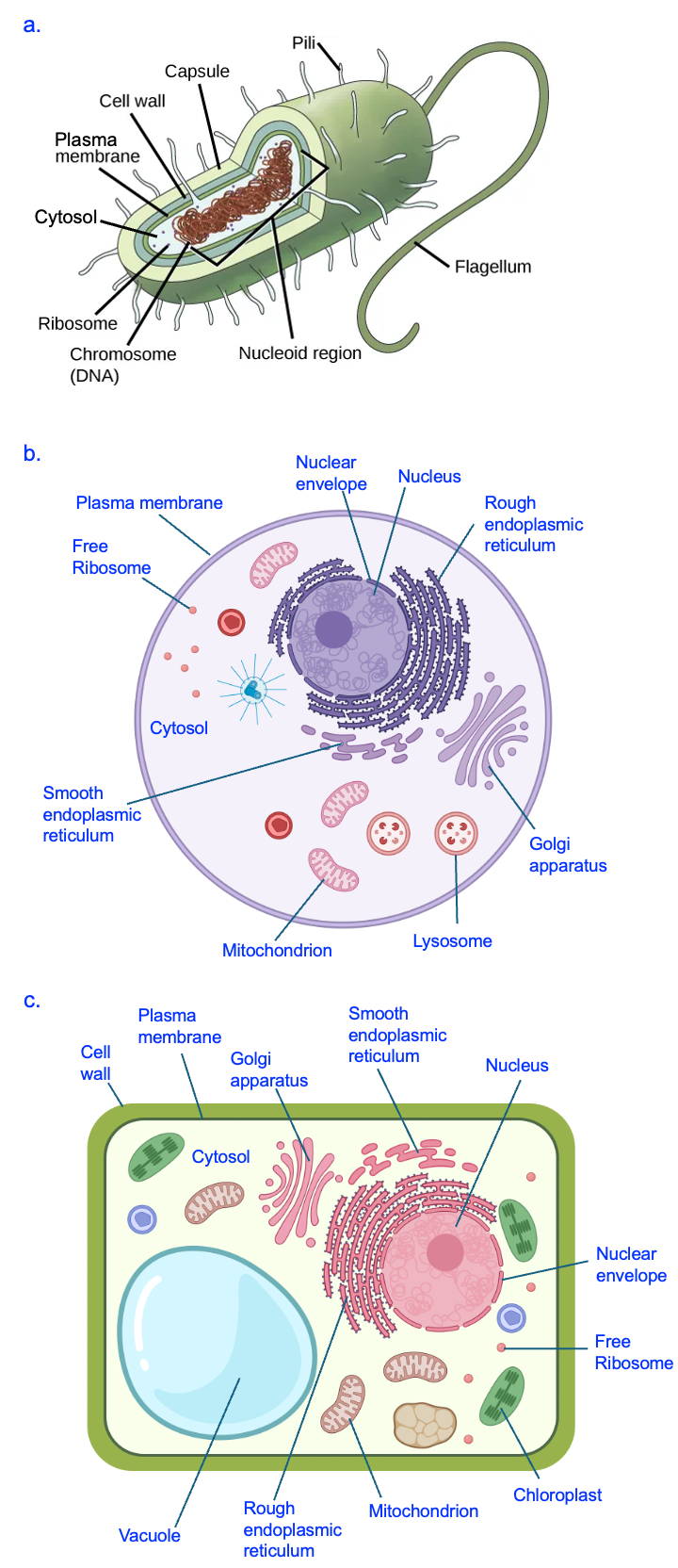
We discussed in Chapter 4 that the lack of a nucleus means that transcription occurs in the cytosol for bacteria but in the nucleus of eukaryotes. Additionally, the structure of genetic material is different between these cell types. While the DNA in eukaryotic cells is found in many linear segments, the genomic material of bacterial cells is organized into a single, circular chromosome (organized into a structure called a nucleoid).
Another feature difference that is likely related to the lack of internal membranes is the relative size of bacterial cells as compared to eukaryotic cells. While extreme examples exist, a typical bacterium is 1–2 microns (micrometers, µm) in size, while a typical eukaryotic cell is at least 10 times larger, 10–100 µm in size. This creates a high ratio of surface area to volume in bacteria and a low ratio in most eukaryotic cells. Finally, the presence of so many internal compartments, created by many internal membranes, means that the total amount of membrane in eukaryotic cells is much higher than in bacterial cells. In subsequent chapters, we will identify further differences between bacterial and eukaryotes.
Section 9.4 Eukaryotic Cell Compartments
Internal compartments are a defining feature of eukaryotic cells, but what potential advantages are conferred by having these compartments? First, compartments can be used to isolate and group proteins and molecules that relate to a particular process. Second, compartments can separate two actions that are in opposition, for example the synthesis of molecules versus the breakdown of molecule. Lastly, these internal membranes increase the surface area available for processes that require a membrane. Membranes and specific membrane proteins are crucial in both cellular respiration and photosynthesis. Cells with more membrane devoted to those processes can produce more products and better manage their energy needs.
With these ideas in mind, let’s explore the specific functions of certain organelles in more detail.
The Nucleus
The nucleus is a prominent organelle with the cell and is where a cell’s genomic DNA is housed and the site of transcription. Another unusual feature of the nucleus is that is has a double membrane (two phospholipid bilayers) called the nuclear envelope. The outer membrane of the nucleus seamlessly connects with the organelle next to the nucleus, the endoplasmic reticulum (more on this organelle below).
Substances that need to enter or exit the nucleus do so through nuclear pores that span both membranes—note the large gaps in the nuclear envelope in Figure 9.6b. Consider the process of transcription. What molecules (proteins and small molecules) would need to enter the nucleus for transcription to occur? What products of transcription would need to exit the nucleus for the next step of gene expression to occur?
Endoplasmic Reticulum
Directly adjacent to the nucleus is the endoplasmic reticulum or ER, which is a series of interconnected tubules. This organelle is involved in both protein and lipid synthesis. As shown in Figure 9.8, the “rough” appearance of the ER is due to ribosomes (red dots) that are attached to the ER membrane, which is the basis of the name rough endoplasmic reticulum. Many ribosomes are also found freely floating in the cytosol, but the ER-attached ribosomes specifically translate proteins right across the membrane of the ER. Proteins synthesized at the ER are typically those that will be secreted from the cell, or function in the plasma membrane. The ER is also the site of translation for proteins that function in the ER itself, in the Golgi apparatus, and the lysosome (described below).
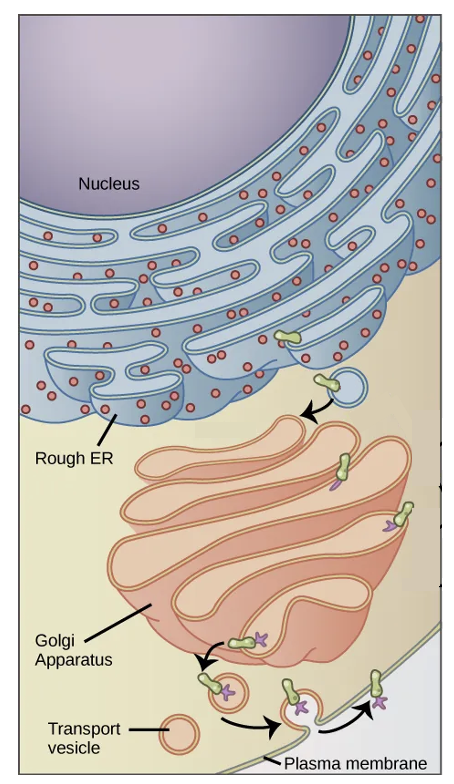
Some parts of the ER do not have any attached ribosomes (see Figure 9.7b). This smooth ER is where lipids are synthesized, including phospholipids for the plasma membrane.
Golgi Apparatus
Proteins and lipids synthesized in the ER are sent to the Golgi apparatus, which is a series of flattened sacs. In Figure 9.8, a protein (green shape) was translated by a ribosome attached to the ER membrane. This membrane with its embedded protein was transferred to the Golgi apparatus, where specific sugars (purples shapes) are added by the process of glycosylation. Lipids from the ER are similarly modified (see Figure 9.3). The sugars added to proteins help the proteins fold correctly and retain their structures even in harsher environmental conditions. Some proteins that later go on to the cell surface are involved in how one type of cell recognizes another type of cell. Glycosylation is also the basis of ABO blood types that you may be familiar with. If you have type A blood and a friend has type B blood, you have different types of sugars present on the surface of proteins on the red blood cells.
Another function of the Golgi is sorting of proteins. It can direct proteins to the plasma membrane or send them to other organelles in the cell, such as the lysosome.
Lysosomes
One example of an organelle that receives proteins from the Golgi is the lysosome, which is a cellular recycling center (Figure 9.9). In the membrane of the lysosome, a transporter protein called a proton pump brings H+ into the lysosome from the cytosol. The higher concentration of H+ in the lysosome reduces the pH, making this compartment much more acidic than the cytosol. Most enzymes function at roughly neutral pH, which is the pH of the cytosol, because this environment allows for the correct protein folding. However, the lysosome has specialized enzymes called acid hydrolases that only function at acidic pH. The proton pumps and acid hydrolase enzymes are examples of proteins that are initially translated by ribosomes attached to the ER and then sorted by the Golgi to the lysosome to carry out their functions.
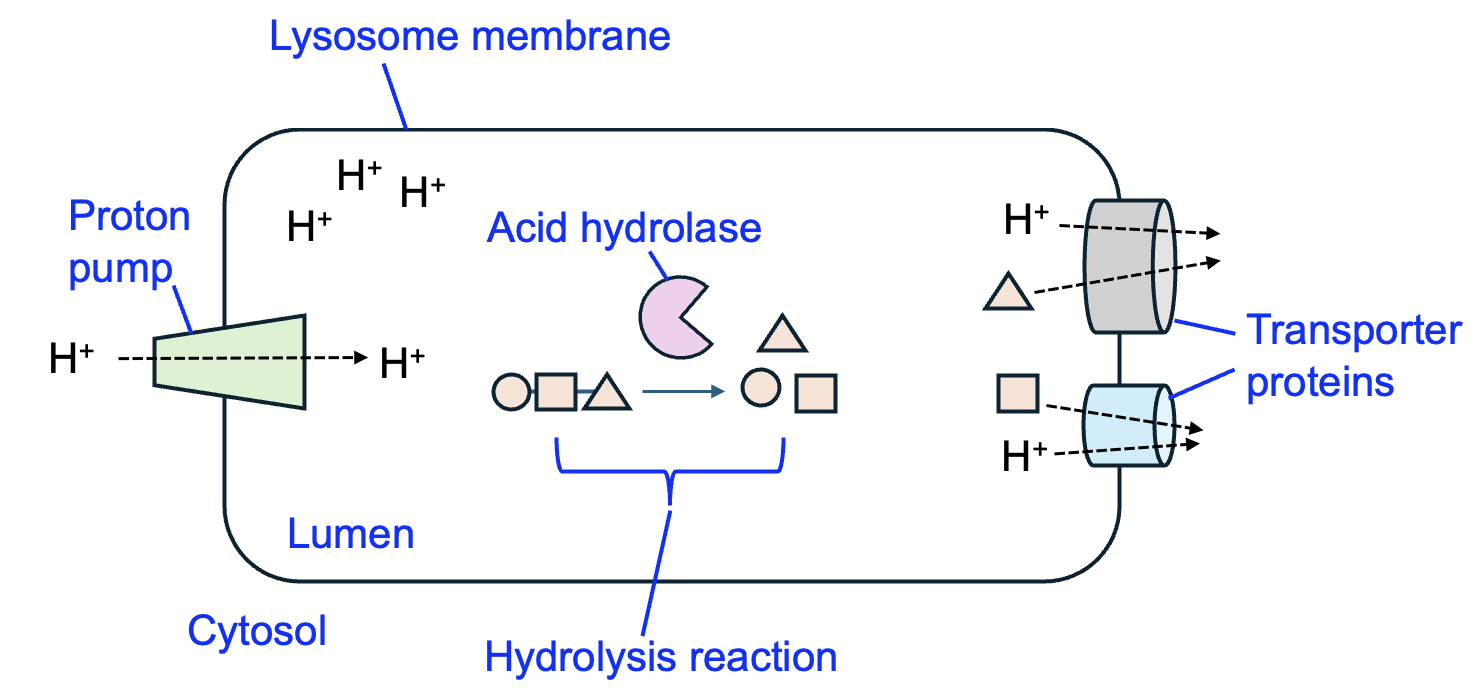
The lysosome receives macromolecules and even organelles from other parts of the cell and breaks them down into their monomer components. In Chapter 3, we discussed how amino acids can be joined to make a protein by reactions that also produce water. In the lysosome, the opposite reaction occurs—acid hydrolase enzymes carry out hydrolysis reactions, splitting a water molecule each time a monomer is removed from the chain. Proteins, lipids, and carbohydrates are examples of large molecules that are broken down by acid hydrolases into their smaller building blocks. To complete the recycling process, additional transporters in the lysosome membrane transport monomers (and H+) into the cytosol, where these monomers can be incorporated into macromolecules once again.
The lysosome illustrates a key advantage of compartments—the ability to separate opposing reactions. Protein synthesis reactions occur in the cytosol, while protein breakdown reactions occur in the lysosome. This is because the enzymes to carry out the reactions are present only in those specific compartments, and acid hydrolase enzymes for the breakdown reactions require the specific pH of the lysosome to carry out their functions.
The lysosome is an organelle found in animal cells, but plant cells have an organelle called a vacuole to break down macromolecules (see Figure 9.7c).
Mitochondria and chloroplasts
Both mitochondria and chloroplasts are essential organelles for cellular energy management. Mitochondria carry out cellular respiration in all types of eukaryotic cells, including plant cells. In a series of reactions, glucose and oxygen are converted to CO2 and water, and the free energy released in these reactions is used to produce ATP, which is a cellular form of energy. Figure 9.10a shows the basic structure of mitochondria, which includes an outer membrane and an inner membrane. The inner membrane is extensively folded to greatly increase its surface area, which is essential to its function in producing large numbers of ATP molecules.
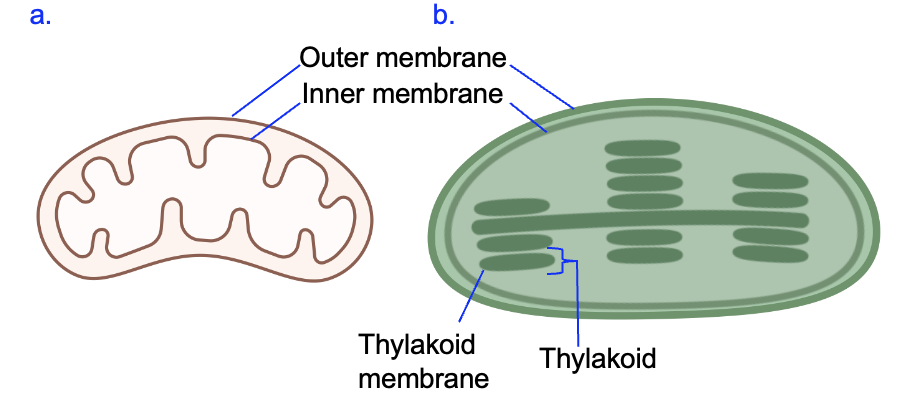
Plant cells also have mitochondria, but they additionally have chloroplasts that essentially carry out the opposite chemical reaction. Plants and other photosynthetic organisms take CO2 and water and use the energy from light to produce carbohydrates such as triose phosphates. These products can then be converted into glucose and other molecules that the cell needs. Figure 9.10b shows that, like mitochondria, chloroplasts have both outer and inner membranes. However, the chloroplast has many smaller compartments called thylakoids, which are created by a special membrane called the thylakoid membrane. We will see later that this membrane is critical for plants to harvest the energy of light. While the details differ, both mitochondria and chloroplasts have membranes with large surface areas that allows them to contain many copies of the proteins needed to harvest energy.
Interestingly, both mitochondria and chloroplasts have their own DNA and can even produce some of their own proteins. This is one of many pieces of evidence that suggest both organelles were originally free-living bacteria. Scientists hypothesize that over billion years ago, these bacteria were engulfed by a proto-eukaryotic cell and became organelles within that cell.

