11 Membrane Transport
Learning Objectives
- Use the membrane model from Membranes and Cell Compartments (Chapter 9) to explain how and why different molecules can or cannot pass through a membrane and why membranes need proteins to be selectively permeable
- Based on the properties of a given solute and concentration gradients, predict the type of transport required to move that solute across a membrane
- Construct a model to show solutes, concentration gradients, and transport proteins in a membrane, and use this model to explain how cells use energetic coupling to transport solutes across membranes and why this is important for cellular function
In Chapter 9, we learned that the plasma member defines the outer border of the cell and helps separate the external environment from the internal components of the cell. Additionally, we saw that eukaryotic cells have additional internal membranes that create compartments within the cell, where specialized functions can occur. As useful as these barriers are, they also create complications—nutrient molecules need to enter and waste molecules need to exit the cell—and most of these molecules cannot efficiently cross lipid bilayers. To solve this problem, cells have many transport proteins embedded in membranes to regulate molecule movements. Molecule transport through these proteins follows the energetics principles from Chapter 10, including equilibrium and energetic coupling.
Chapter Outline
Section 11.1 Selective Permeability of Membranes
Section 11.2 Passive Transport Processes
Section 11.3 Active Transport Processes
Section 11.1 Selective Permeability of Membranes
While membranes create barriers, they aren’t impenetrable walls. Based on their structures and properties, some molecules cross membranes very easily (the membrane is permeable to those molecules), while the membrane blocks the movement of other molecules. To help predict which molecules would fall into each category, first review the structure of the membrane. All cell membranes are a lipid bilayer of phospholipids (Figure 11.1). The heads of the phospholipids are polar and face the exterior or interior of the bilayer. Meanwhile, the nonpolar fatty acid tails face each other and form the core of the membrane.

Any molecule that crosses the membrane must be able to interact with the fatty acid tails. Thus, the membrane is permeable to molecules that are primarily nonpolar (Figure 11.2). These include gases, such as O2 and CO2, and steroid hormones, such as testosterone and estrogen.
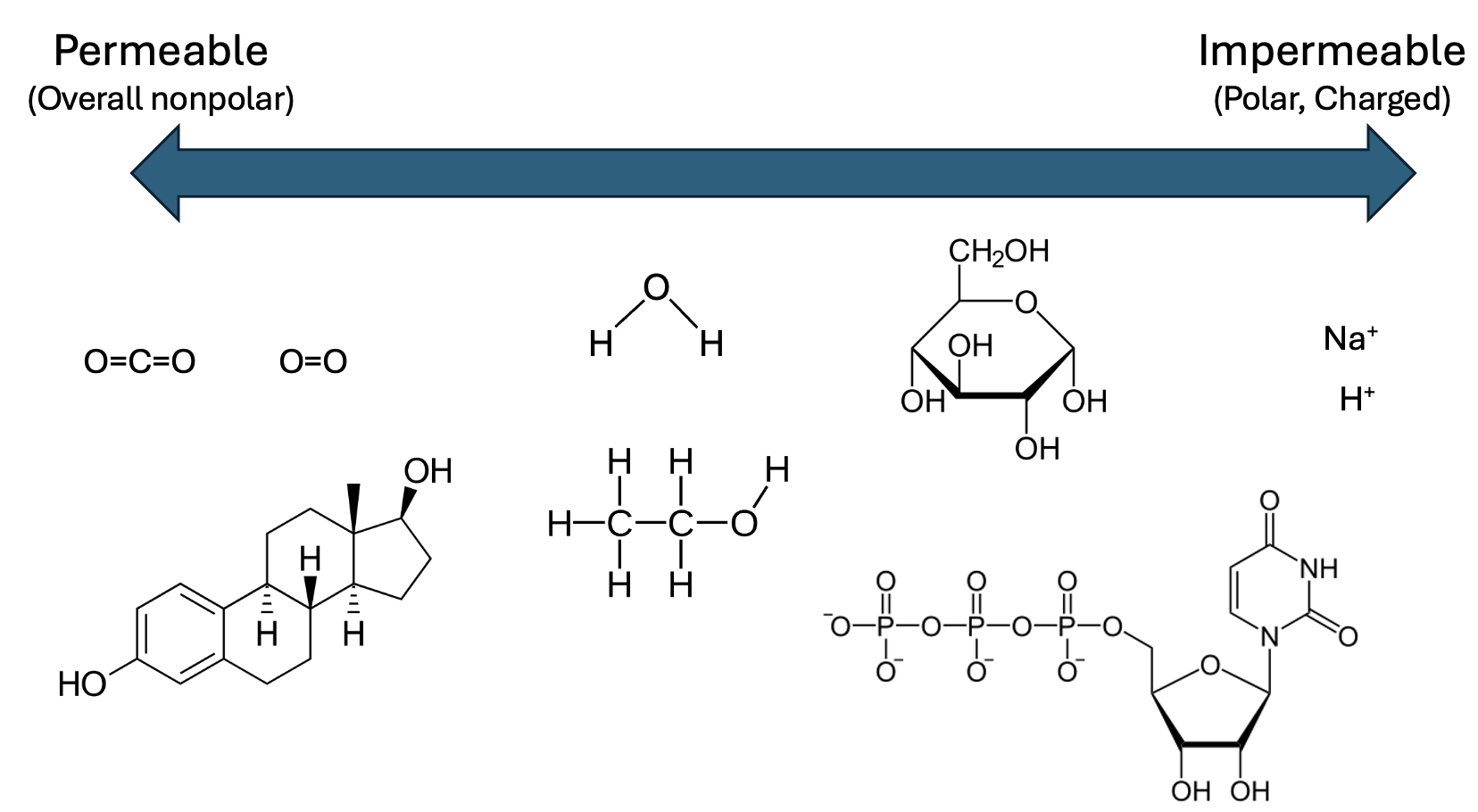
Molecules that are more polar, such as H2O and ethanol, have limited permeability. In the case of H2O, this permeability does not allow sufficient movement for biological systems. Finally, the membrane is impermeable to strongly polar or charged molecules, such as nucleotides, amino acids, or ions. Thus, cells require transporter proteins that span the lipid bilayer to move most substances from one compartment to another.
Section 11.2 Passive Transport Processes
In Chapter 10, we noted that molecule movements in a specific direction can either release energy as the system progresses towards equilibrium or require energy to move the system farther from equilibrium. In the cell model shown in Figure 11.3a, there are more red circle molecules inside the cell versus out.
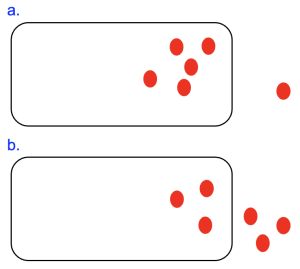
Thus, movement of these molecules out of the cell shown in Figure 11.3a would be energetically favorable, as this is movement from an area of high concentration to low, with the concentration gradient. Molecule movements of this type, which do not require energy input, are also referred to as passive transport.
There are several different subcategories of passive transport, which vary depending on the molecule moved, whether a transport protein is required to facilitate the transport, and the structure of the transport protein if required. Regardless of the subtype, all passive transport processes depend on diffusion, which is the random movement of molecules. For uncharged solutes, if there is a concentration gradient as shown in Figure 11.3a, the net direction is with the concentration gradient, moving out of the cell.
Simple Diffusion
Let’s imagine the red circle molecule is small and nonpolar. In this case, the lipid bilayer is permeable to the molecule and type of transport in this case is simple diffusion. Over time, we would expect that molecule concentrations would even out across the membrane, as shown in Figure 11.3b. Note that even when this equilibrium state is reached, molecule movements continue. However, there is no longer a net movement in one direction over the other.
Simple Diffusion Simulation
Use this simulation to explore the effects of concentration in simple diffusion:
https://lab.concord.org/embeddable.html#interactives/sam/diffusion/5-permeable-membrane.json
Created by The Concord Consortium, https://concord.org.
Facilitated Diffusion: Passive Transport through Channels and Carriers
A lipid bilayer is permeable to a few molecules only, and most molecules that enter or exit the cell require the assistance of a transport protein. Let’s imagine the red circle molecule in Figure 11.3a is an ion, like Na+ or K+. Even though it is energetically favorable for the ion to exit the cell, the lipid bilayer is impermeable to charged molecules and thus no ion movement occurs under this condition. However, membranes are permeable to ions if they contain a type of transporter called a channel (Figure 11.4a).
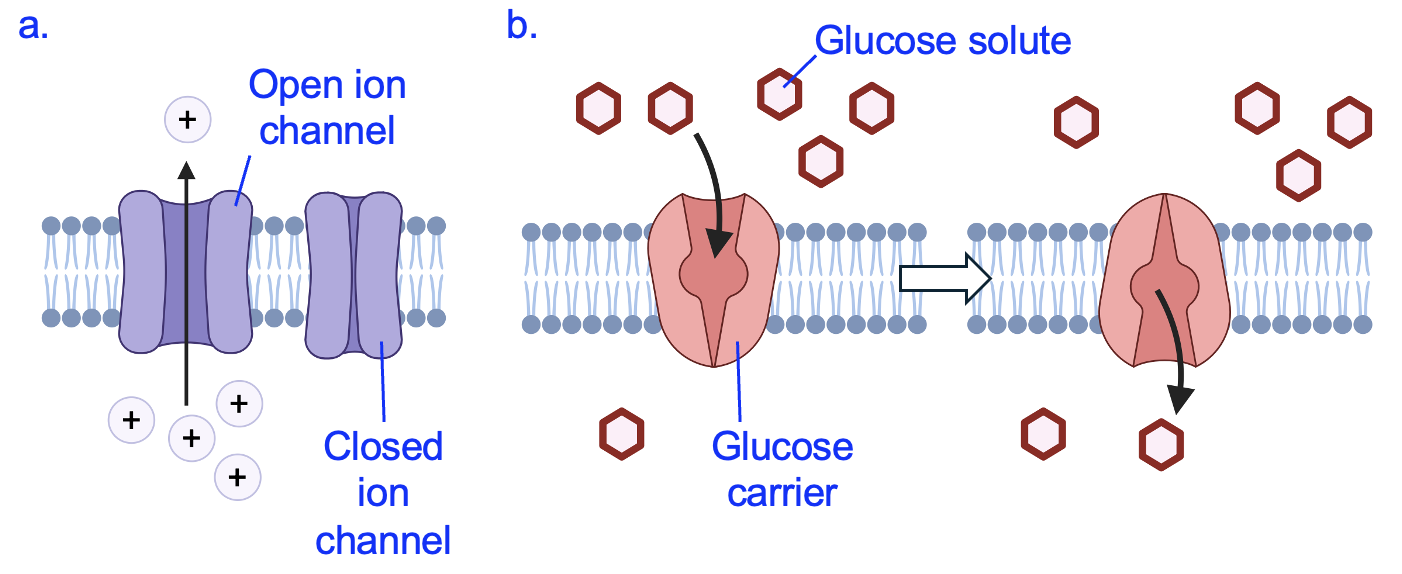
Like all transport proteins, channels create a hydrophilic path across the hydrophobic lipid bilayer. A selective pore in the channel allows only certain ions to pass through based on size and chemical properties (such as positive or negative charge). Only certain types of cells have ion channels, meaning the movement of ions across cell membranes is generally restricted to cells that need them to carry out their functions. For example, in the membranes of neurons and muscle cells, ion channels that open and close under precise conditions are critical for communicating information and causing muscle contractions to occur.
Another molecule that requires a transport protein is glucose, which we will soon see is essential for cells to create ATP. Glucose has many polar covalent bonds that prevent it from crossing the membrane via simple diffusion (Figure 11.2). A carrier protein for glucose (Figure 11.4b) is open on one side of the membrane at first. After the carrier binds a molecule of glucose, the protein changes shape to open on the opposite side to release its bound glucose. Like enzymes, carrier proteins only bind to certain molecules, which makes them selective in the molecules they can transport. The overall direction of movement through this carrier is determined by the concentration gradient. If there were more glucose molecules in the lower compartment of Figure 11.4b, net glucose would be transported in the opposite direction, towards the top compartment.
Passive Diffusion Simulation using Channels
Facilitated Diffusion: Osmosis
A special form of passive transport concerns water. While small, water is also polar enough that it has limited permeability through lipid bilayers (Figure 11.2). However, the plasma membranes of many cells have proteins called aquaporins. These “water channels” allow for rapid volumes of water to enter or exit cells. This has important consequences in situations when the movement of solutes is restricted.
Figure 11.5 shows a beaker of water with solutes (small circles) at the start (left image) and after time has passed (right image). The dashed red line indicates a membrane that is permeable to water but not the solutes, and more solutes are present on the right side of this membrane than on the left. Initially, the volumes are the same on both sides of the membrane, but over time the volume on the right side increases and the concentration of solute molecules appears similar in both compartments. What caused this change?
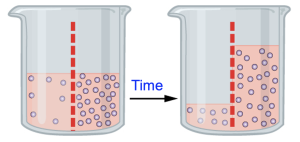
Initially, the concentration gradient across the membrane means it is energetically favorable for solutes to move from the right compartment to the left. But because the membrane is not permeable to solutes, the solutes are trapped on the right side of the membrane.
However, this membrane is permeable to water, and water moves following the rules of all passive transport processes. In this figure, the water molecules are not drawn, but we can reason that a solution of equal volume with more solute molecules has fewer water molecules. Effectively, where the solute concentration is high, the water concentration is low and vice versa. Therefore, it is energetically favorable for water to move from the left side of the beaker to the right, since this is moving from an area of high to low water concentration. A useful description of this is “water follows the solutes.” This movement of water to balance solute concentrations is called osmosis.
Again, most cells have aquaporin proteins that allow the movement of water into and out of the cell. To prevent excess movement of water, cells need to regulate their internal concentrations of solutes so that they match as close as possible the external environment. Figure 11.6 shows red blood cells in three different environmental conditions.

The middle image of Figure 11.6 shows a condition in which red blood cells are in a solution with similar solute concentrates as the cytosol of the cells (isotonic). A red blood cell in isotonic solution has the typical structure of a flattened disc with a depression in the middle (also called biconcave). Moreover, although water moves in and out of cells through aquaporins, there is no net movement as solute concentrations (and water concentrations) are the same inside and out.
However, if solute concentrations outside the cell became more concentrated, perhaps due to dehydration, water leaves the cell. As a result, the cell becomes shriveled and does not function as effectively. The leftmost image of Figure 11.6 shows red blood cells in this hypertonic solution.
Finally, if the solute concentration outside the cell is much lower than inside the cell, water enters the cells. If only a small amount of water enters, the cells swell and lose their flattened disc shape, as shown in the rightmost image in Figure 11.6. However, if the external solution has much lower solute concentrations, much more water enters the cells, causing the cells to lyse, or burst. This is a key reason why treatments that are given intravenously (injected into the bloodstream) are dissolved in isotonic saline solution and not water!
Section 11.3 Active Transport Processes
Up to this point, we have only given examples of molecules moving in energetically favorable directions, with their concentration gradients. These movements do not require any energy input as they bring systems toward equilibrium. However, there are many cases in which cells need to move molecules in ways that are energetically unfavorable, such as to import and concentrate nutrients, or to maintain specific solute concentrations. All these efforts require energy input and energetic coupling similar to the reaction coupling discussed in Chapter 10. However, rather than two chemical reactions being coupled, here we will see examples of favorable chemical reactions coupled to unfavorable molecule movements, or favorable molecule movements coupled to unfavorable molecule movements. Regardless, the principle remains the same—using a protein to link something that provides energy to something that requires energy, resulting in an overall energetically favorable process.
Primary Active Transport
In a typical animal cell, Na+ concentrations are much higher outside the cell, while K+ concentrations are much higher inside the cell. These conditions are no accident—animal cells expend significant energy moving Na+ out and bringing K+ in to create these conditions. We will see in a later section how the cell utilizes these unequal ion concentrations to do useful work.
Figure 11.7 shows the special type of carrier protein called the sodium-potassium ATPase (Na+-K+ ATPase) that creates these unequal concentrations of Na+ and K+ across the plasma membrane. This pump moves Na+ out of the cell, from an area of low concentration to high, while also moving K+ into the cell, from an area of low concentration to high. Thus, both movements are energetically unfavorable.
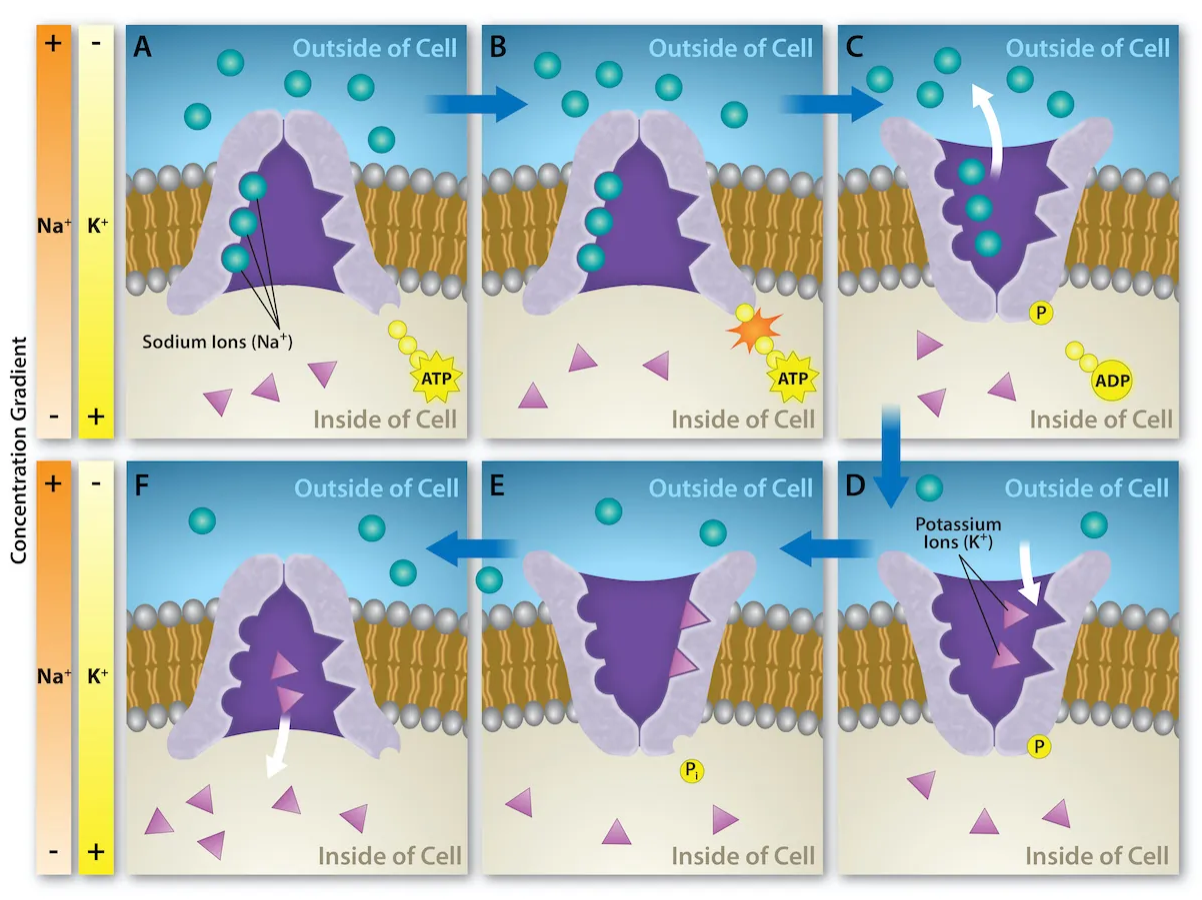
The source of energy coupled to these molecules movements is a reaction involving ATP. Because an energy input is utilized to move molecules in an energetically unfavorable direction, this is called active transport. Furthermore, because ATP is the energy source, this is called primary active transport (you can rearrange the initials for Primary Active Transport to get ATP).
As shown in Figure 11.7a, the pump is initially open facing the cytosol of the cell. Once three Na+ bind, the protein breaks off a phosphate from ATP, producing ADP (Figure 11.7b). The phosphate attaches to the pump, causing it to change shape and open towards the outside of the cell and the Na+ ions are released into the extracellular fluid (Figure 11.7c). Next, two K+ in the extracellular fluid bind (Figure 11.7d). The phosphate is released from the pump (Figure 11.7e), causing it to change shape and reopen again facing the cytosol and the K+ ions are released (Figure 11.7f). This cycle repeats, moving 3 Na+ out and 2 K+ in, using ATP in each cycle and creating non-equilibrium conditions across the plasma membrane.
Secondary Active Transport
As shown in Figure 11.2, membranes are generally impermeable to ions. Unless specific transport proteins are present and open, ions stay in the compartments where they are found. The actions of the Na+-K+ ATPase create high concentrations Na+ outside the cell. Those ions will remain outside the cell unless there is a protein transporter that allows them to enter the cell. This concentration gradient across the membrane is an energy source that can be utilized to move other molecules.
An analogous process is a dam on a river. When closed, the dam blocks the flow of water, leading to accumulation of water on upstream side. However, when the dam is opened, water rushes through, releasing large amounts of energy that can be harnessed as hydroelectric power.
In a cell, the favorable movement of Na+ through a protein can be energetically coupled to the unfavorable movement of other molecules into or out of the cell. Figure 11.8 shows a carrier protein that moves glucose and Na+ into a cell. Notice that the concentration of Na+ is higher outside the cell, while the concentration of glucose is higher inside the cell. Thus, the import of Na+ is energetically favorable, while the import of glucose is energetically unfavorable.
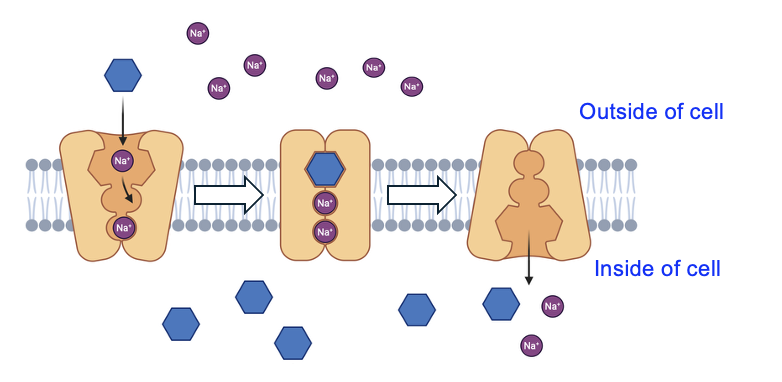
The transporter is first open to the exterior, and as Na+ is more abundant outside of the cell, Na+ usually binds first to the transporter. However, the transporter remains in this position until both Na+ and glucose bind. The binding of both molecules causes the transporter protein to change shape and open on the inside of the cell. After the Na+ and glucose are released into the cytosol, the transporter resets to be open on the outside of the cell.
Notice how each cycle of the Na+-glucose transporter brings Na+ into the cell. This would eventually cause Na+ concentrations to reach equilibrium across the membrane, were it not for the continuous work of the Na+-K+ ATPase to export Na+. This is just one of many transporters in animal cells that uses the Na+ gradient created and maintained by the Na+-K+ ATPase. The type of transport carried by the Na+-glucose transporter is called secondary active transport, due to the dependency on the primary active transport carried out by the Na+-K+ ATPase.
Plant and bacterial cells use a similar process to transport molecules across the plasma membrane, against their concentration gradients. First, a proton pump uses ATP to move protons (H+) into the extracellular space, creating a concentration gradient where proteins are more concentrated outside of the cell. Then, the favorable import of protons is coupled to the unfavorable movement of other molecules.

