2 Biological Chemistry
Learning Objectives
- Compare and contrast covalent bonds and intermolecular forces
- Describe intermolecular forces and their relative strength in aqueous environments
- Given the names or structures of two functional groups, predict the most relevant intermolecular force between them
Cells need to sense their environment, respond to signals, and maintain their interval environment, among other tasks. Amazingly, cells do this without the eyes, ears, and ability to speak that we might use to do these same things. Instead, cells use chemistry to sense and respond to chemical signals. Because of this, cells must follow the rules of chemistry, and we need to explore the chemical basis of biological molecules in cells to understand and make predictions about the behavior of these molecules and cells more broadly.
Chapter Outline
Section 2.2 Overview of Interactions in Cells
Section 2.4 Noncovalent Intermolecular Forces
Section 2.6 Drawing Organic Molecules
Section 2.1 Atomic Structure
Cells are the smallest unit of life and are made up of molecules. In turn, molecules are made up of atoms, which are a unit of matter. An atom has a nucleus that typically contains protons and neutrons, with electrons that orbit the nucleus (Figure 2.1).
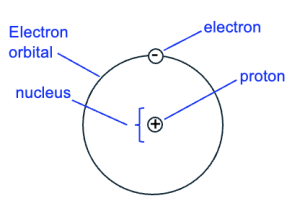
Each proton has a positive charge, each electron has a negative charge, and neutrons have no charge. If the number of protons in an atom equals the number of electrons orbiting the nucleus, the atom overall has no charge.
The Bohr model of the atom shows electrons arranged about the nucleus in electron shells, with electrons filling the shells innermost shells first. Depending on numbers of electrons, different atoms have different numbers of electron shells. Models of the atoms commonly found in biological molecules are shown in Figure 2.2.
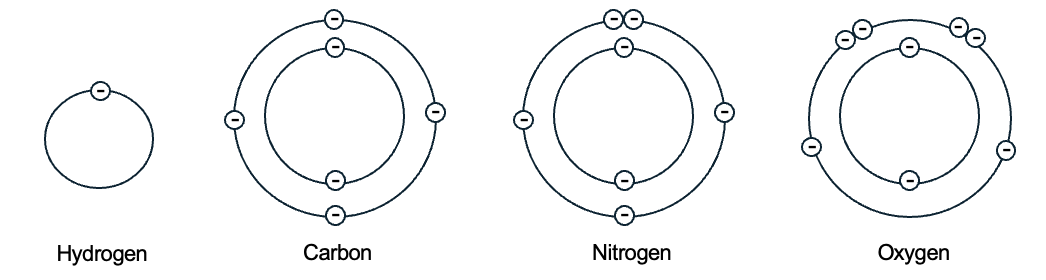
Note that a hydrogen atom has one proton and one electron, and only one electron shell. In contrast, carbon has six protons, six neutrons, and six electrons. Two electrons are found in the inner electron shell, and the remaining four electrons are in the outer shell of carbon. In subsequent sections, when we discuss interactions between atoms, our focus will be on the electrons in the outermost shell of each atom, called valence electrons. It is the number of valence electrons that influences the number and type of interactions that atoms have with other atoms.
Section 2.2 Overview of Interactions in Cells
A bond is an interaction between atoms. When bonds form, energy is released into the surrounding environment, and the atoms themselves achieve a lower energy state. In later segments of this chapter, we will introduce several different types of interactions, which we can divide into two broad categories: covalent bonds and intermolecular forces. Covalent bonds are formed when atoms share valence electrons (“co-” refers to sharing, “-valent” refers to valence electrons). Covalent bonds link atoms of a molecule together, so, these bonds are intramolecular (within a molecule) bonds. For example, water is a molecule with two hydrogens and one oxygen, and covalent bonds link each hydrogen to an oxygen atom. In contrast, the forces described later are often between molecules, or intermolecular.
To quickly grasp the relative strength of these different biologically relevant interactions, the graph in Figure 2.3 shows the maximal bond energy in aqueous solution.
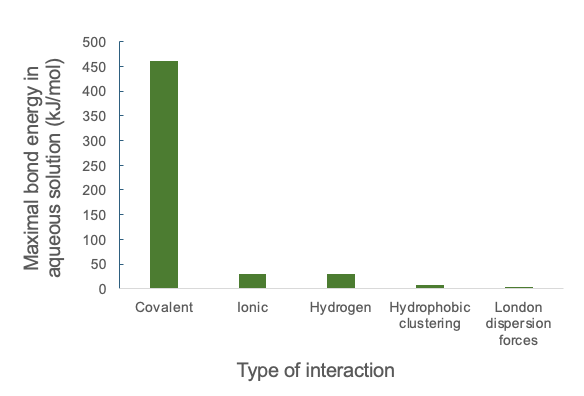
The strength in aqueous solution, as opposed to strength in a vacuum as you might have seen in chemistry, is used because biological systems are aqueous. Bond energy can be defined as either the amount of energy released when an interaction occurs, or the amount of energy input required to break an interaction. One observation of the graph is that covalent bonds release significantly more energy when they form, shown by the much taller bar as compared other forces. Thus, covalent bonds can endure for long periods of time, while London dispersion forces may only last a few femtoseconds (fs, or 10-15 s).
Section 2.3 Covalent Bonds
When atoms can fill their outermost shells with electrons, they are more stable. A hydrogen atom needs just one more electron to fill its outermost electron shell (which can hold two total electrons), while a carbon atom needs four more electrons (for a total of eight electrons in its outer shell). These requirements can be satisfied if one carbon atom bonds with four hydrogen atoms. When atoms interact by sharing electrons, they form a molecule. If one pair of electrons is shared, this is a single bond. However, two or three pairs of electrons can be shared, forming double or triple bonds, respectively. In the molecule methane, CH4, there are a total of four covalent bonds, one single covalent bond between the carbon atom and each of the four hydrogen atoms (Figure 2.4a). The shared electron pairs are easily visualized in the top representations of these molecules, but we will more commonly show molecular structures, using a solid line between atoms to indicate a covalent bond, as shown below.
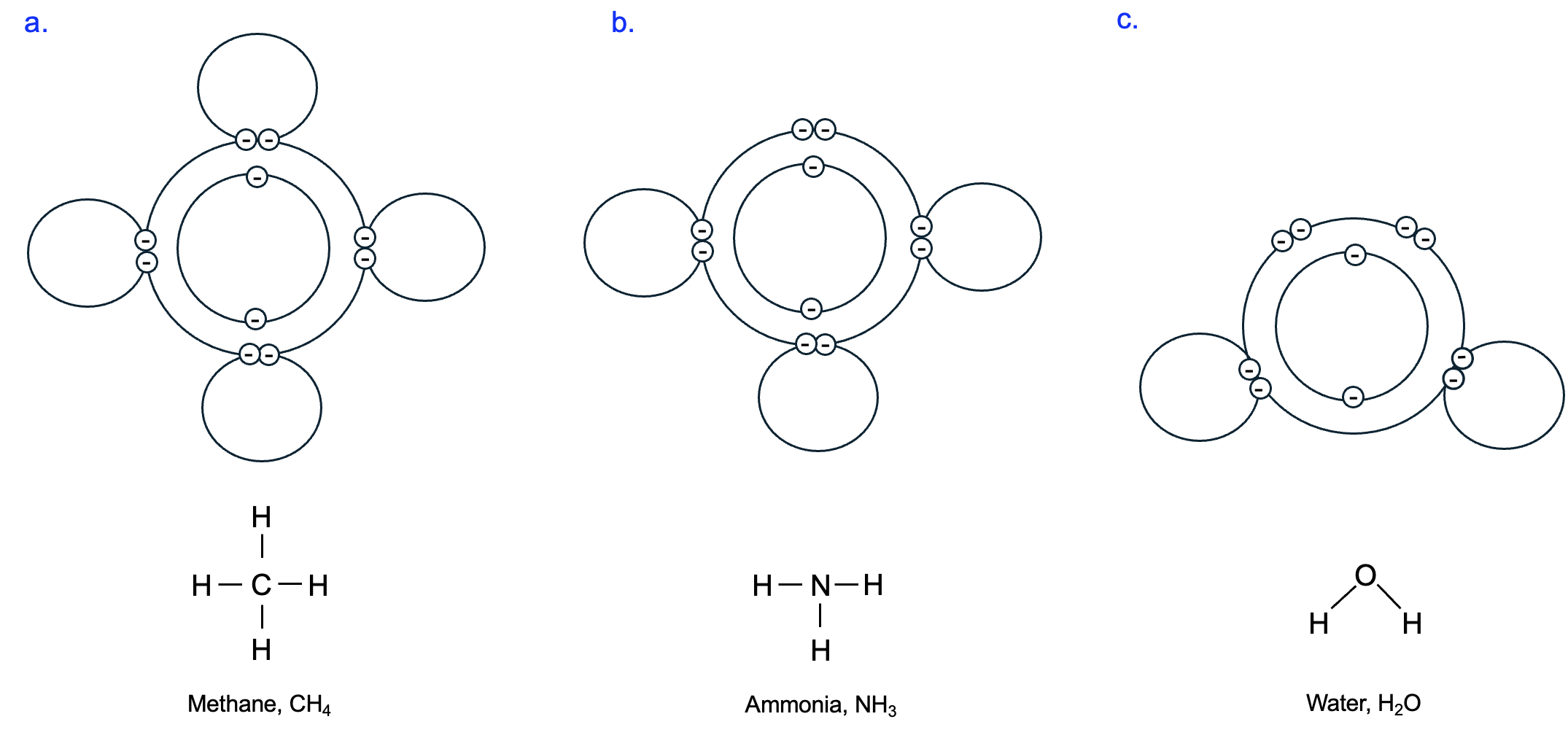
Following this same pattern, nitrogen has five electrons in its outer shell and thus forms three bonds with other atoms to fill its outer shell, and oxygen with its six electrons in its outer shell forms two bonds with other atoms (Figure 2.4b). For a molecule of water, H2O, there are two covalent bonds between a single oxygen atom and two hydrogen atoms (Figure 2.4c). In later chapters, we will discuss the chemical reactions that occur to form or break covalent bonds.
While the bonds within the methane and water molecules are all strong covalent bonds involving the sharing of electrons, there are distinct differences in how these electrons are shared due to the elements that are sharing electrons. Atoms of different elements vary in their electronegativity, or how strongly the atoms attract electrons. As a general rule, elements on the top right area of the periodic table have the highest electronegativity values. For our purposes, we will focus on elements that are most common in biological molecules: carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). Of these four elements, O is the most electronegative, followed by N, then C and H, with C and H being similar in electronegativity.
What are the consequences of electronegativity for bonding? Well, when atoms with high electronegativity, such as oxygen, are bonded with atoms of lower electronegativity, such as hydrogen, the electrons are not equally shared within the covalent bond. Because electrons have a negative charge, the association of those atoms with the more electronegative atom causes it to have a permanent partial negative charge (indicated by δ– symbol). Consequently, the bonded atom with the lower electronegativity (lower electron affinity) has a permanent partial positive charge (indicated by δ+) since electrons associate less with that atom. When there is a charge difference across a covalent bond, due to unequal electron sharing, this bond is called a polar covalent bond (Figure 2.5a). However, if electrons are shared equally, as is the case when carbon is bonded to hydrogen, there is no charge difference and the bond between these atoms is called a nonpolar covalent bond (Figure 2.5b). Likewise, when any atom is bound to another atom of the same element, as in the case of molecular oxygen, O2, this is also a nonpolar covalent bond. Note that partial charges are rarely drawn in molecular structures, but you should learn to recognize when they are present and draw these yourself. A good rule of thumb is that molecules with O and N are likely to have polar covalent bonds, given the high electronegativity of both O and N.
Section 2.4 Noncovalent Intermolecular Forces
As we can see in Figure 2.3, many types of weak forces can form between atoms or molecules that do not involve sharing of electrons (i.e., are noncovalent). Instead, electrostatic (charge-based) interactions are the basis of the intermolecular interaction. For all of these, remember that opposite charges are attractive, and like charges repel.
Hydrogen Bond Interactions: a Type of Dipole-Dipole Interaction
In the structure of water (H2O), recall that the O covalently bound to H has a stronger affinity for the electrons that it covalently shares with the two H. Thus, each atom on water has a partial charge. When there are many molecules of water, interactions between molecules can be stabilized if an H (with its partial positive charges) is near an O atom of another water molecule, since that O has a partial negative charge (Figure 2.5).

Remember, opposite charges (full or partial) are attractive. The interaction in this case is called a hydrogen bond interaction, and each water molecule can form multiple hydrogen bonds with other water molecules. Notice how many parallel lines (a dashed line may also be used) indicate a hydrogen bond interaction between molecules, which is different from the solid lines used to indicate covalent bonds. The many intermolecular interactions formed between water molecules are key for some special properties of water that you may be familiar with, such as the ability of water to form droplets and to be in liquid phase at room temperature.
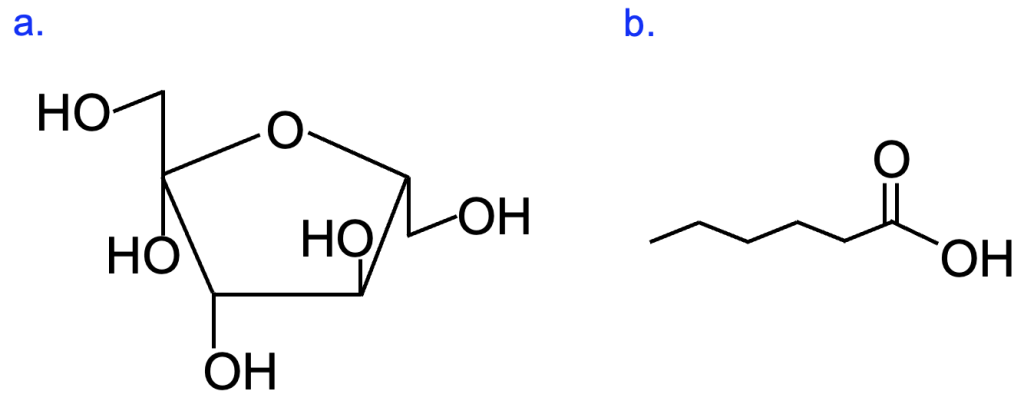
Hydrogen bonds can form between molecules other than water as well. Consider the molecule shown in Figure 2.6. What atoms are bonded together in this structure? Are these polar or nonpolar covalent bonds?
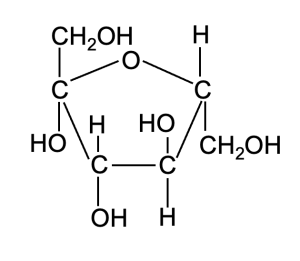
Notice how the molecular structure contains many bonds between O and H, same as we see in water. Therefore, the bonded O and H atoms have the same partial charges as we observed for these atoms in a molecule of water. Molecules such as those shown in Figure 2.6, in which there are many polar covalent bonds, are considered polar molecules. You can often identify polar organic molecules by looking for oxygen or nitrogen bonded to carbon or hydrogen, as oxygen and nitrogen are much more electronegative than carbon or hydrogen, and thus shared electrons in the covalent bond are not equally shared. Molecules with polar covalent bonds can form hydrogen bonds with water and with other polar molecules—the only requirements are a hydrogen with a partial positive charge in one molecule to interact with a partial negative charge on another molecule. A broader term for this type of interaction between opposite partial charges in different molecules is dipole-dipole interaction. A hydrogen bond is a type of dipole-dipole interaction that is very abundant in biological systems. Although individually much weaker than covalent bonds, these interactions can be very influential in large numbers. In fact, the hydrogen bond interactions between water molecules are what causes water to be liquid at room temperature, when similarly-sized molecules (for example, H2S) with less ability to form intermolecular hydrogen bonds are gases.
Ionic Bond Interactions
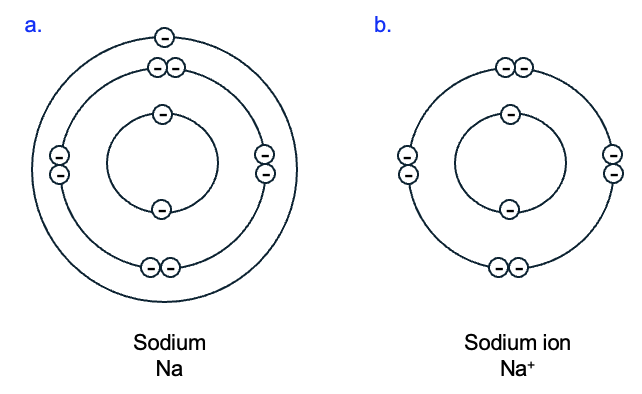
To achieve a full outer electron shell, instead of forming covalent bonds, some atoms give up or gain protons or electrons, resulting in atoms with unequal numbers of protons and electrons. For example, a sodium atom (chemical symbol Na) has 11 protons and 11 electrons, with only a single electron in its outermost shell (Figure 2.7a). If the sodium atom loses that outermost electron, how many protons does it have, compared to electrons? Having lost an electron, it is now an ion with a positive charge, written Na+) (Figure 2.7b). Ions are always designated with + or – symbols to indicate full charges, and a number to indicate the number of full charges if more than one.
As we saw with hydrogen bonding, the interaction of oppositely charged molecules is stabilizing. Figure 2.8a shows portions of two biological molecules, one with a full positive and one with a full negative charge, interacting with an ionic bond interaction. Notice how each molecule retains its full charge even as the interaction occurs between the opposite charges.
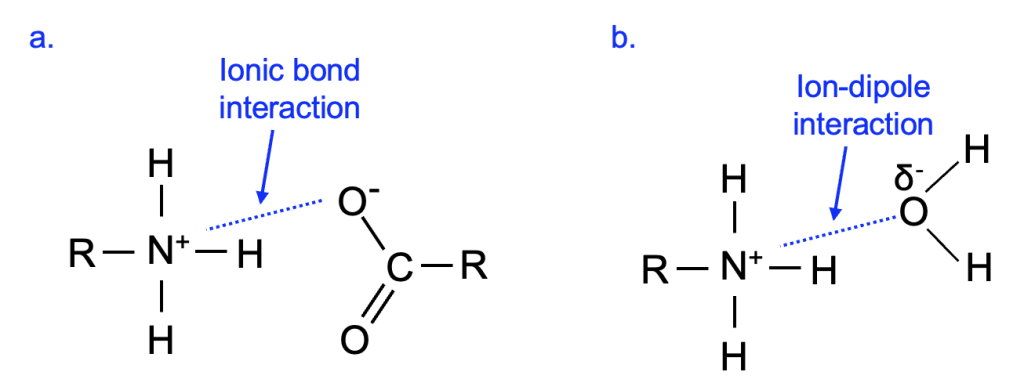
While the absolute strength of a given interaction is in part dependent on the context, a reasonable general assumption is that interactions between two fully charged molecules are stronger than interactions between two partially charged molecules. What if there is a full positive charge in one molecule and partial negative charge in another molecule? These can interact with an ion-dipole interaction, and the strength of that interaction is roughly intermediate between an ionic interaction (opposite full charges that interact) and a dipole-dipole interaction (opposite partial charges that interact) (Figure 2.8b). In all cases, polar or charged molecules can be referred to as hydrophilic (“water loving”) as they interact readily with water.
Hydrophobic Clustering (Hydrophobic Effect)
Thus far, we have seen examples of many types of noncovalent interactions between polar molecules. But what about nonpolar molecules? Consider the molecule in Figure 2.9.

As discussed earlier, C and H are similar in electronegativity, so the bond between them is a nonpolar covalent bond with no partial charges. Without either full or partial permanent charges, these atoms cannot form ionic or dipole interactions, and thus are not strongly attracted to polar molecules that can interact in these ways. In fact, if you try to mix nonpolar molecules (like oil, which are similar in structure to the molecule in Figure 2.9) with polar molecules (like water), you can observe that these molecules rapidly separate into two layers (Figure 2.10).

The detailed reasons for this phenomenon are complex, so we will focus on the broad outcome: nonpolar molecules appear to avoid water (they are hydrophobic, or “water fearing”), and instead are observed to cluster together and away from water or other polar molecules. This has the effect of minimizing the interface between polar and nonpolar molecules and is called hydrophobic clustering or the hydrophobic effect. Unlike the other noncovalent interactions in this chapter, this is not an attractive interaction among nonpolar molecules, but an outcome of the properties of water and other polar molecules. Despite this, we group this phenomenon with noncovalent interactions for organizational simplicity. As shown in the graph, the energy required to disrupt this interaction is weaker than most interactions or forces. Nevertheless, since cells (and biological systems more broadly) contain a lot of water, hydrophobic clustering has a strong influence on the behavior of biological molecules and the organization of cells.
London dispersion forces
The weakest type of intermolecular force is the London dispersion force (LDF), often referred to as van der Waals interactions in biology textbooks. Recall that every atom has a nucleus containing positively charged protons, and negatively electrons that orbit the nucleus (Figure 2.1). The specific region where electron cloud is located as electrons orbit has a transient partial negative charge, with a corresponding area of transient partial positive charge found where the electron cloud is not. When two molecules are brought very close together, areas of transient partial charges in one molecule can induce changes in the electron cloud density of atoms in the other molecule, created an induced dipole. These regions of partial positive and partial negative charges can interact for very weak LDFs to occur between the molecules. Because LDFs are individually very weak and transient, they are most influential when molecules are close together, the structure of the molecules allows many interactions occur between molecules (remember, there is strength in numbers!), and when stronger attractive interactions are not possible. Thus, while LDFs occur between all types of molecules that are close together, LDFs are relevant mainly for nonpolar molecules. We will see examples of this in the next chapter when we discuss lipids.
Test your knowledge of interactions!
Section 2.5 Solubility
Imagine you have a spoonful of table salt (NaCl) and a spoonful of sugar. Both are white, crystalline substances, and when added to water, both seem to “disappear” or dissolve into the water. Both of these examples illustrate a key property of water, that of being an excellent solvent for substances that contain partial or full charges. But why is this, and is the same thing happening in each case?
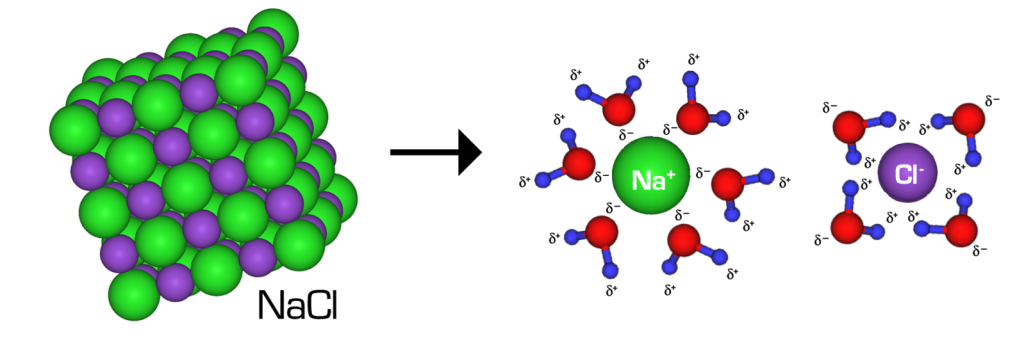
If we could observe this at the molecular level, we would see two very different things happening. Table salt consists of Na+ and Cl– ions—what type of interaction occurs between these ions? Ionic bonds between Na+ and Cl– keep the ions together in the absence of water, and this interaction is quite strong under these conditions. However, when NaCl is added to water, the ions separate as water molecules surround, or solvate, each ion, forming numerous ion-dipole interactions (Figure 2.11). In right side of the figure, notice how the partially negative oxygen of water is oriented towards the positively charged Na+, but away from the negatively charged Cl–. This solvation occurs because the bond between Na+ and Cl–, while strong in the absence of water, is easily overcome by the sheer number of water molecules and interactions they form with the ions.
Similarly, a sugar crystal contains many molecules of sugar interacting with each other. Review the structure in Figure 2.6—if there were multiple molecules, what interaction occurs between them? Hopefully you noticed that this structure contains many oxygen atoms bonded to hydrogen atoms, which we know is are polar covalent bonds. Therefore, sugar molecules can form hydrogen bonds with other sugar molecules in the absence of water. What happens when sugar is dissolved in water? Based on the NaCl example, you might have expected that the bonds within the sugar molecule would be broken. However, remember that the bonds within sucrose are strong, covalent bonds that require a large amount of energy to break, even in aqueous solution (Figure 2.3). The weaker hydrogen bonds between sugar molecules, however, are easily overcome with new interactions with water. So the covalent bonds within the molecule remain intact, but hydrogen bonds with water replace the hydrogen bonds between sugar molecules.
Section 2.6 Drawing Organic Molecules
One definition of organic molecules is that they contain at least one carbon-carbon or carbon-hydrogen bond, and often there can be many of these bonds in organic molecules. Due to the abundance of these atoms, scientists have developed a shorthand for drawing organic molecules. In this method, carbon atoms are indicated by the ends of lines and vertices where two lines meet, unless another type of atom is shown there. Hydrogen atoms bonded to carbon are usually not drawn either. However, we know that carbon forms four covalent bonds, and we can assume that any bond to carbon that is not otherwise indicated is a hydrogen. Figure 2.12 shows two molecules that were discussed earlier in this chapter in this simplified format. Compare Figure 2.12a with Figure 2.6, and Figure 2.12b with Figure 2.9. While this way of drawing structures is often confusing at the start, it greatly speeds up the drawing of these molecules.
Section 2.7 Functional Groups
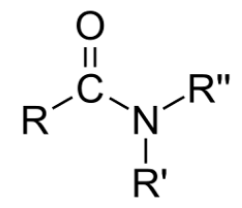
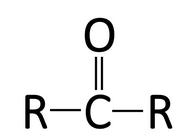
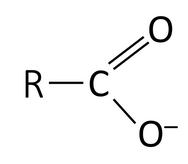
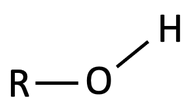
A functional group is a specific group of atoms that are attached to a larger molecule, and they often determine key properties of those molecules, such as how they will behave in water, and give those larger molecules the ability to chemically react. The key functional groups we will encounter on biological molecules are shown, with their structures, in Table 2.1. Because functional group names are part of the vocabulary for the course, and we will refer to them often to describe biological molecules, you should create flash cards to practice recognizing and drawing them.
Notice that most functional groups contain oxygen or nitrogen covalently bonded to hydrogen or carbon. Based on our earlier discussion of electronegativity, this tells us that most functional groups contain polar covalent bonds. Which functional group is the exception? Knowing that, think about what interactions can each of these functional groups have if they are part of a molecule.
Importantly, some functional groups change their properties based on the pH of the surrounding solution. pH, as you may recall from earlier courses, is a measurement of the concentration of protons in a solution. As an important aside, when we say “protons,” we might draw as a charged hydrogen, or H+. A hydrogen atom is one proton and one electron (Figure 2.1), and if the electron is lost, now we just have a proton—that’s where the H+ comes from. pH values can range 0–14, with 7 being neutral pH. Pure water has a pH of 7. A pH lower than 7 indicates an acidic substance, corresponding to higher proton concentrations in the solution. Therefore, substances that increase proton concentrations in the surrounding environment are said to be acidic. Conversely, higher pH values reflect lower proton concentrations, and any molecule that absorbs protons from the solution and decreases proton concentrations is said to be basic.
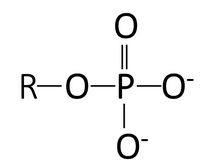
Some functional groups on molecules act as acids at the pH of the cell. Look at the structures of carboxyl and phosphate in the first column of Table 2.1, noting that both groups have O bound to H. At the pH of the cell, however, one or more of these protons are donated to the surrounding environment. The electrons are left behind, and therefore each O has one more electron than they have protons, resulting in a negative charge. Thus, carboxyl and phosphate groups function as acids at the pH of the cell, decreasing the pH of the surrounding solution. The right column of Table 2.1 shows these groups as having one (carboxyl) or two (phosphate) negative charges, as they typically appear at the pH of the cell.
In contrast, the amino functional group, which is nitrogen bound to two hydrogens, often acts as a base at the pH of the cell. A base does the opposite of an acid—instead of donating protons, it takes up protons from the solution. When the amino group gains a proton, it creates a positive charge on the N. Note—not every amino group will take up protons from the environment. It depends on the context of the rest of the molecule that amino group is found. To be clear, we will generally draw amino groups with their charges if in that context they are present. In the case of carboxyl and phosphate functional groups, you can assume that at the pH of the cell, these are in their charged form, regardless of how they are drawn.
Exercises
Test your knowledge of functional groups!
a unit of matter
a negatively charged component of an atom
electrons in the outermost shell of each atom
an interaction between atoms
an interaction between atoms in which those atoms share valence electrons
two or more atoms joined by covalent bonds
occurring within a molecule
occurring between molecules
the amount of energy released when an interaction between atoms occurs, or the amount of energy input required to break the interaction
a process that forms and/or breaks covalent bonds
how strongly an atom attracts electrons
a bond in which electrons are unequally shared due to one atom being more electronegative than the other
a bond in which electrons are equally shared due to both atoms having similar electronegativity
an interaction between a hydrogen with a partial positive charge in one molecule and an atom with a partial negative charge in another molecule
the interaction of opposite partial charges in different molecules
an atom or molecule with one or more full charges
an interaction between opposite full charges
an interaction between a fully charged ion and a dipole with the opposite partial charge
a molecule that interacts readily with water and other polar solvents
molecules do not interact well with water but instead interact with nonpolar solvents
the tendency of nonpolar molecules to avoid water
the inverse log of the concentration of protons in a solution
having a higher concentration of protons (H+) than pure water, corresponding to a pH value lower than 7.
having a lower concentration of protons (H+) than pure water, corresponding to a pH value greater than 7.

 or
or