14 Photosynthesis
Learning Objectives
- Describe the principles of photosynthesis, including the transformations of energy and matter that occur in the process
- Use a model to explain how changes to metabolic substrates or products in one part of the process affect other parts of the process
- Use a model of photosynthesis to predict how photosynthesis is affected by mutations to genes encoding photosynthesis proteins or chemicals
- Use a model to explain the connections between photosynthesis and cellular respiration in a plant cell
All organisms depend on food molecules like sugars and fatty acids to generate ATP. But what process creates those food molecules, and where does the energy for this come from? In broad strokes, the process for creating food is the opposite of the process used to break down food (Figure 14.1).
In photosynthesis, CO2 and H2O are combined to produce a 3-carbon sugar, triose phosphate, then further reactions to this product are required to make glucose or other food molecules. The anabolic pathway to produce G3P requires energy input in the form of light, which is captured in the production of ATP (from ADP and Pi) and NADPH (from NADP+). in the early reactions of photosynthesis. In addition to producing sugar molecules that can be converted to other organic molecules, photosynthesis notably produces O2 gas. Thus, we can say that photosynthesis is the source of all the food we eat and all the air we breathe.
Chapter Outline
Section 14.1 Overview of Photosynthesis
Section 14.4 Metabolic Connections within Plants
Section 14.1 Overview of Photosynthesis
In some key respects, photosynthesis is the opposite of cellular respiration. Yet, in other ways, these processes have a lot in common. For example, like cellular respiration, photosynthesis is a two-stage process (Figure 14.2).
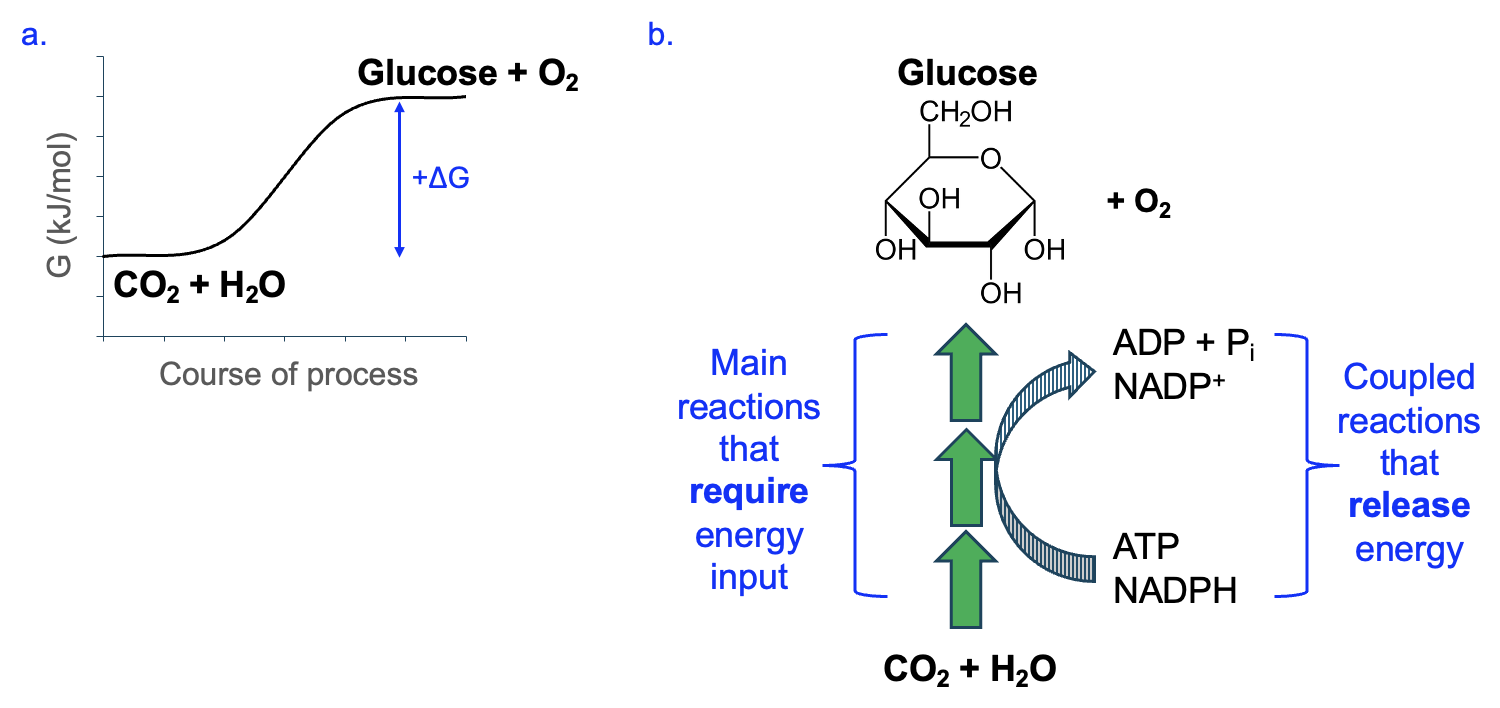
Unlike cellular respiration, the electron transport step (Light Reactions) occurs first in photosynthesis. Like cellular respiration, the formation of energy management molecules captures energy from some reactions that then contribute to later steps. Unlike cellular respiration, the energy management molecules are used to build up carbon molecules: the reactions of the Calvin cycle transform CO2 gas into G3P, a triose phosphate that can be converted into glucose. Like cellular respiration, the light reactions of photosynthesis produce ATP. Unlike cellular respiration, which produces ATP for use throughout the cell, the ATP produced in photosynthesis is used in photosynthesis. Just as most reactions of cellular respiration take place in the specialized compartments of the mitochondria, the reactions of photosynthesis occur in their own compartment created by membranes called the chloroplast (Figure 14.3).

Unlike cellular respiration, which occurs across several cell compartments, all reactions of photosynthesis occur within the chloroplast. In addition to outer and inner chloroplast membranes, another set of membranes creates the thylakoid compartment, and the thylakoid membrane is the site of electron transport and ATP synthesis. On your own, as you go through this chapter, consider more similarities and differences between these major metabolic pathways.
Section 14.2 Light Reactions
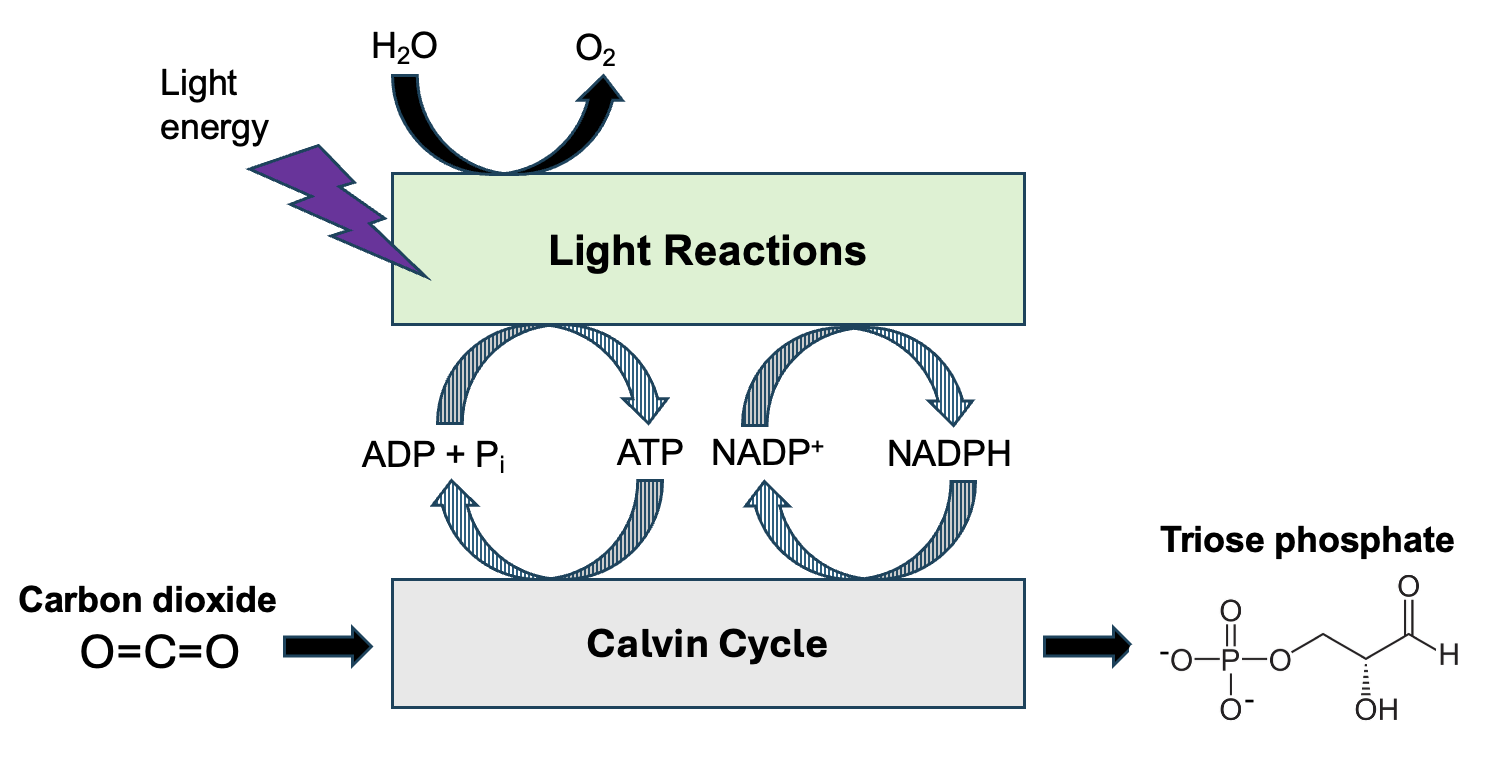
The light reactions of photosynthesis capture light energy and use it to produce ATP and NADPH. This happens in the thylakoid membrane, which is a membrane inside the inner chloroplast membrane, that creates a compartment called the thylakoid lumen (Figure 14.4). The compartment just outside the thylakoid, enclosed by the inner chloroplast membrane, is the stroma. The photosynthetic electron transport chain consists of many proteins embedded in membranes or located just adjacent to the membrane.
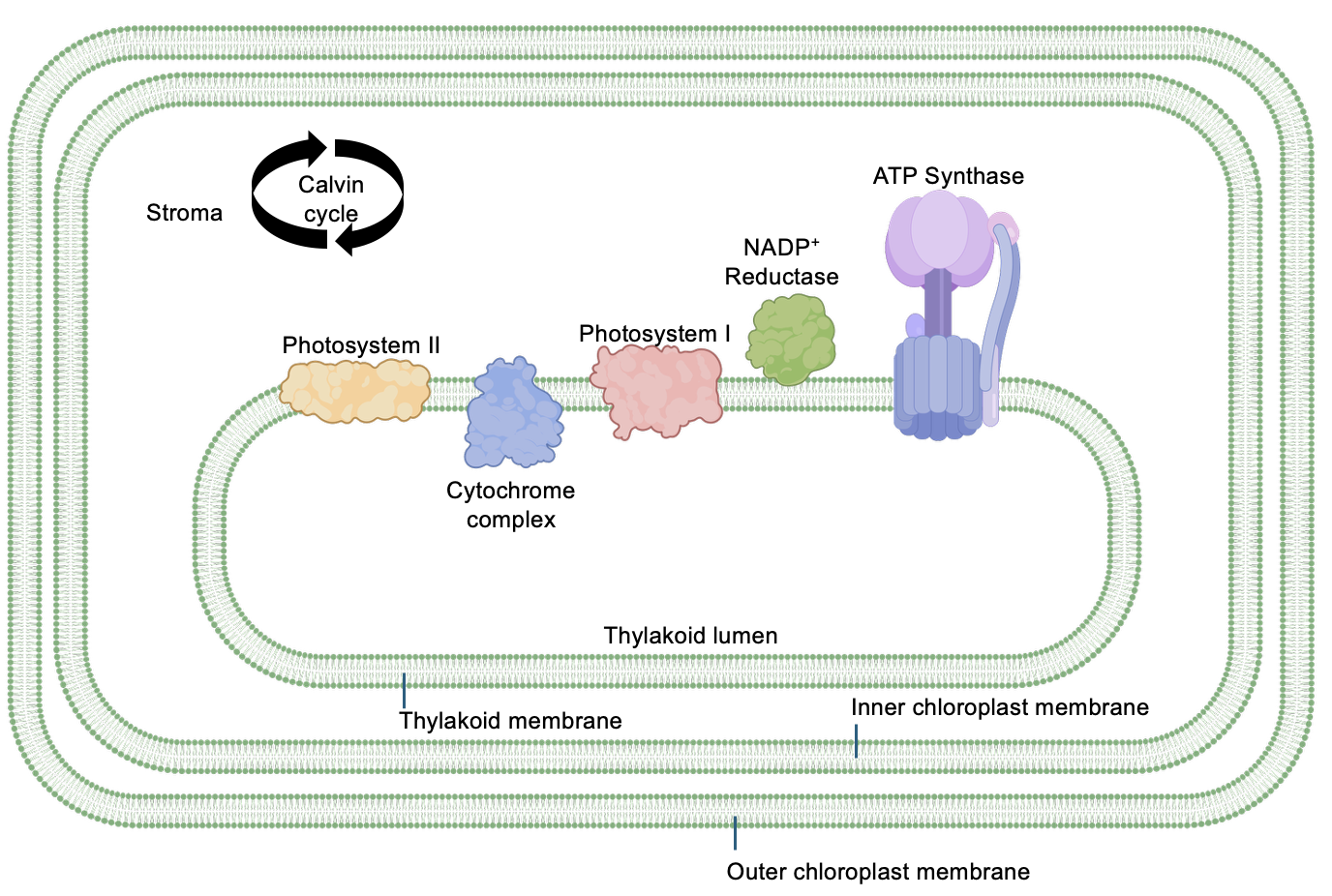
As implied by their names, Photosystem II (PS II) and Photosystem (PS I) are the light-harvesting proteins, able to capture the energy from photons of light and transfer electrons. In between the photosystems is the Cytochrome complex (Cyt), which is both an electron transport protein and a proton pump. Several other molecules that serve as electron carriers between these proteins are not shown for simplicity. The last protein in the chain is NADP+ reductase, which transfers electrons from the electron transport to NADP+, reducing it to NADPH. Another key protein in the thylakoid membrane is ATP Synthase, which is structurally and functionally very similar to the protein in mitochondria.
The photosystems capture the energy of light using a special pigment molecule called chlorophyll, which also gives plants their green color (Figure 14.5a). The complex ring structure at the top of the structure, with its many alternating double and single bonds and magnesium (Mg2+) ion in the center, is the part of the molecule that absorbs light energy. The long hydrocarbon tail allows this molecule to reside in the thylakoid membrane.
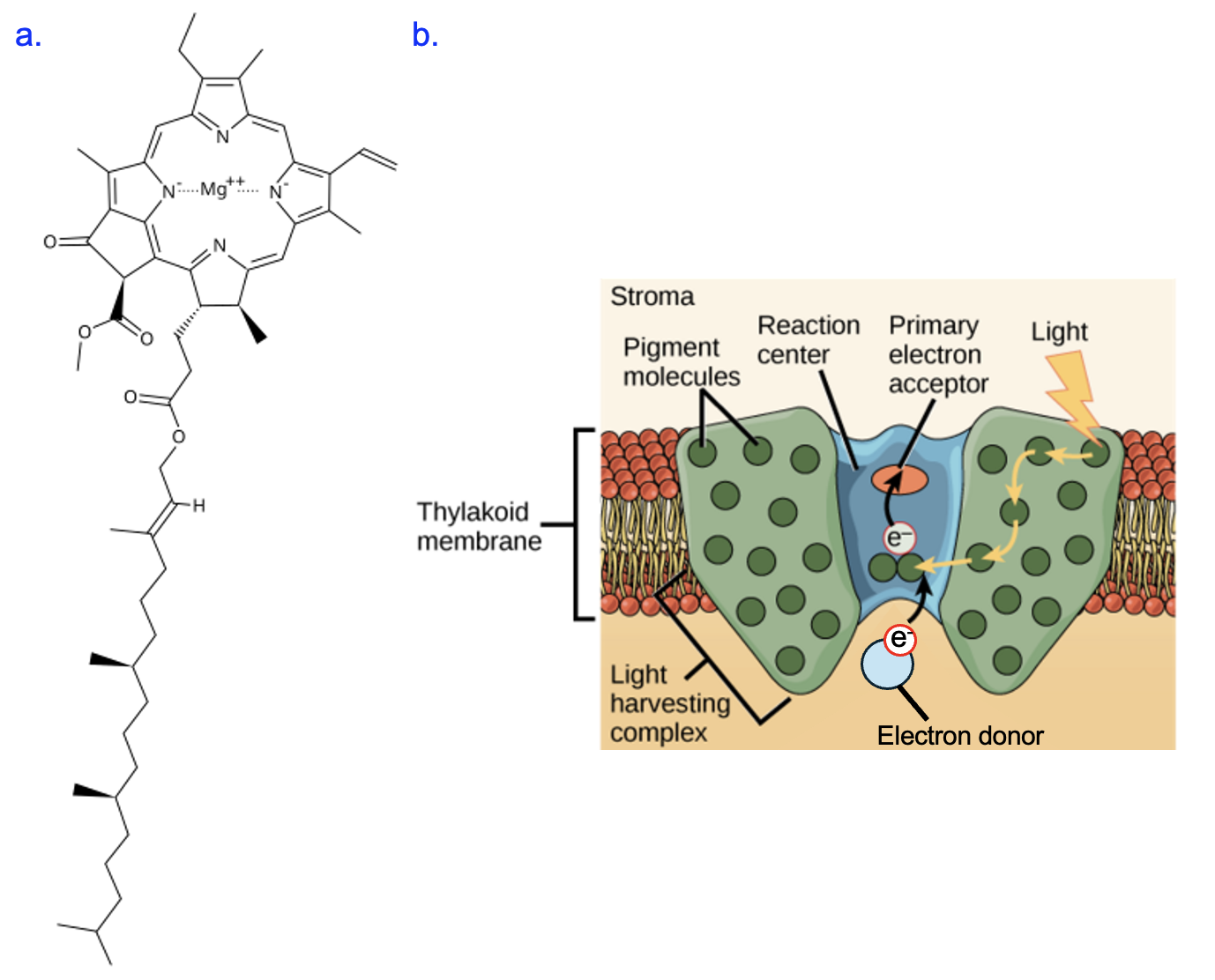
Each photosystem contains many of these chlorophyll molecules and other pigments (Figure 14.5b). When photons strike chlorophyll, electrons in chlorophyll absorb some of this energy and are excited from their ground state. As these electrons return to their ground state, some energy is transferred to neighboring chlorophyll molecules. Due to the structure of the photosystem, this transferred energy is funneled to two special chlorophyll molecules at the reaction center. The convergence of energy onto these molecules causes the chlorophyll to oxidize and pass an electron to an electron acceptor in the photosynthetic electron transport chain.
The first photosystem in the chain is PS II (Figure 14.6). The counterintuitive name is because it was discovered second, after PS I, even though it comes first in the electron transport process. Light input to PSII excites the chlorophyll in the reaction center, causing electrons to be oxidized and go through electron transport.
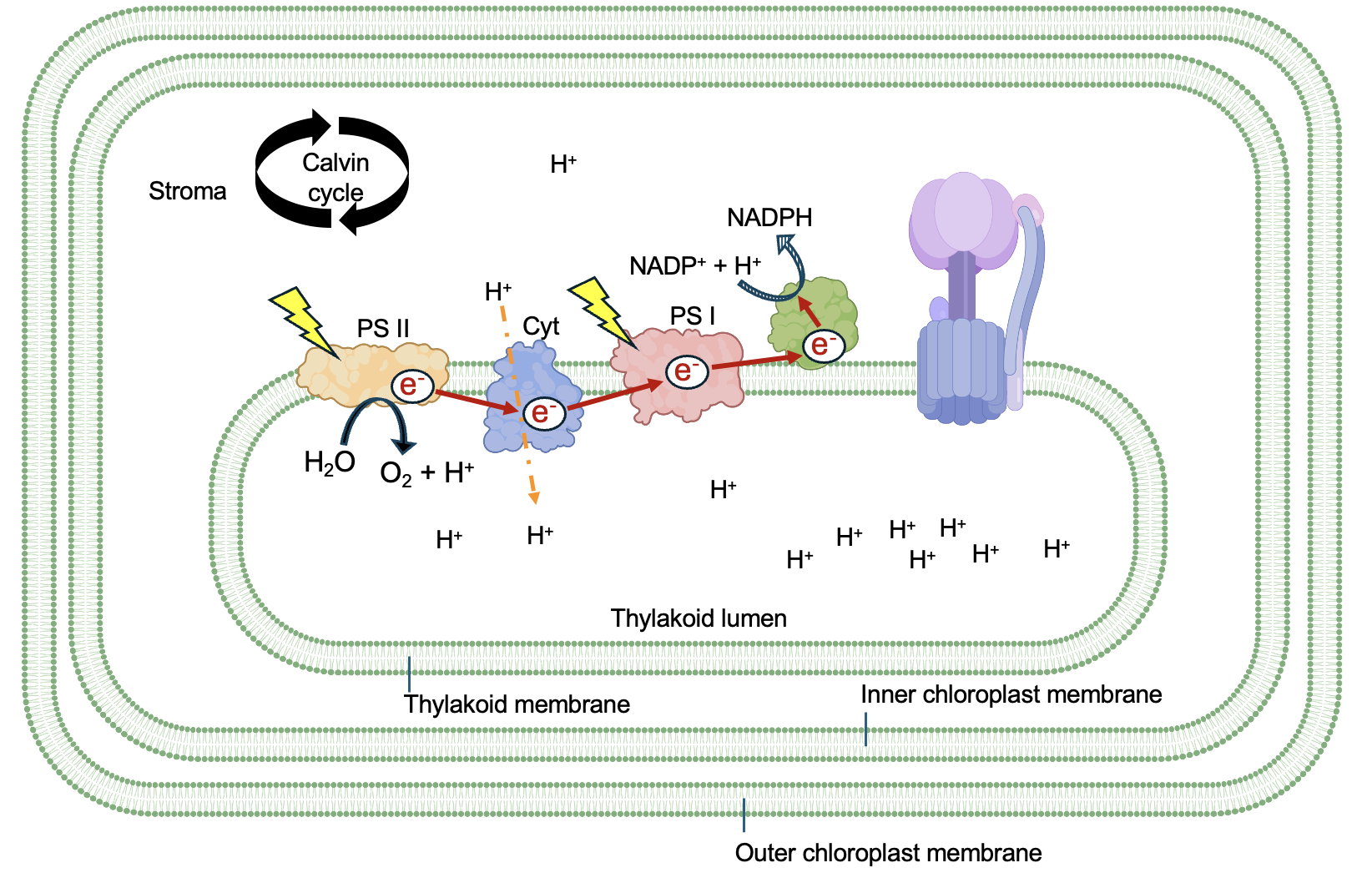
Once oxidized, the lost electron needs to be replaced before more light energy can be absorbed. Within PS II, an enzyme splits water into protons, electrons, and O2. The electrons from water replace those lost in the reaction center chlorophyll, and are thus considered the primary electron input for the photosynthetic electron transport chain. The O2 is a by-product of this reaction, and the protons contribute to a concentration gradient across the thylakoid membrane that we will discuss later.
What kind of reaction happens as H2O is converted to O2? Remember, oxidation is loss, reduction is gain. In this case, H2O is oxidized to O2, which means that the oxygen atoms that were in polar covalent bonds with hydrogen now find themselves in nonpolar covalent bonds. Remember that oxygen has a high affinity for electrons, so it takes a large input of energy to oxidize oxygen, even partially. While the oxidation reactions highlight thus far have all been energetically favorable, this is an example of an oxidation that is very energetically unfavorable. However, oxidized chlorophyll has an even stronger affinity for electrons!
As shown in Figure 14.6, electrons from PS II are transferred to Cyt and ultimately to PS I via redox reactions. This movement is energetically favorable, and the energy released as electrons pass through Cyt is coupled to the movement of protons from the stroma to the intermembrane space. In PS I, another input of light energy energizes the electrons once again, allowing them to be transferred to the final protein in the chain NADP+ reductase. This enzyme captures the energized electrons, using them to reduce NADP+ to NADPH. Energetically, this is very different from the mitochondrial electron transport chain, where electrons associated with NADH have high free energy, which decreases as electrons move through the complexes to eventually form H2O, which has low free energy.
Thus, the movement of electrons through the photosynthetic electron transport chain serves two purposes. First, to contribute to the production of NADPH, the electron carrier. Second, to provide energy to move protons into the lumen of the thylakoid. Notice that the concentration of protons is higher in the lumen than in the stroma (Figure 14.6), indicating this movement is energetically unfavorable. Importantly, two other processes further increase the concentration gradient across the thylakoid membrane: the water-splitting reaction releases protons in the lumen, while the NADPH reaction uses protons in the stroma. Altogether, this means that in full daylight, when light reactions are at their peak, there can be 1000 times more protons in the lumen versus in the stroma.
You might be able to predict how these non-equilibrium protons concentrations might be harnessed by the cell! Indeed, the same process that occurs in mitochondria also happens in the chloroplast: protons move through ATP Synthase from the thylakoid lumen into the stroma, with their concentration gradient. The rotation and movement of subunits with ATP Synthase powers the energetically unfavorable synthesis of ATP (Figure 14.7).
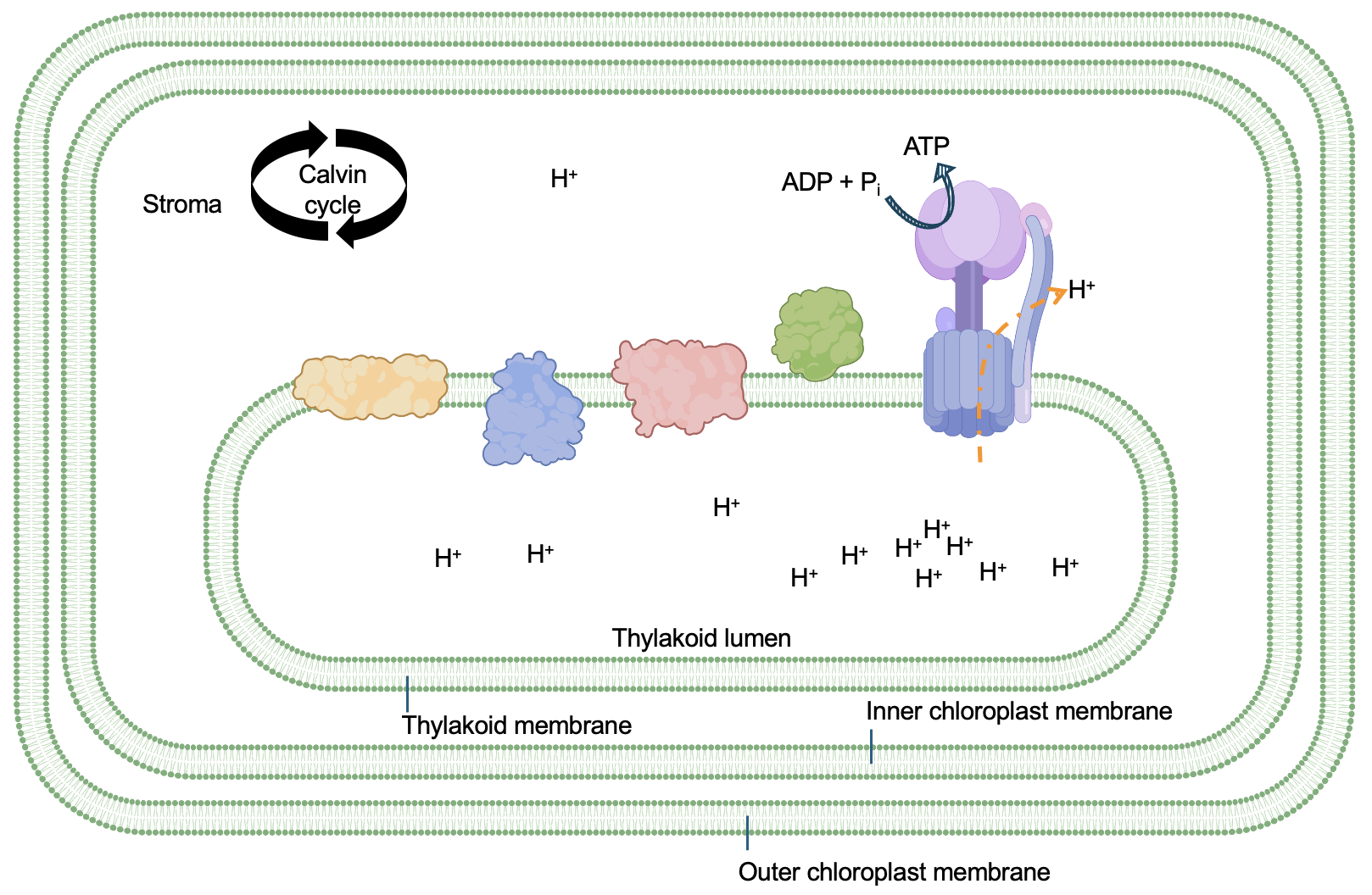
As shown in Figure 14.6 and Figure 14.7, and summarized in Figure 14.2, O2, NADPH, and ATP are products of the light reactions. While O2 is simply a by-product of the light reactions, the NADPH and ATP will contribute to the Calvin cycle, providing much-needed energy for the reactions to convert CO2 to G3P. Conveniently, the Calvin cycle reactions occur in the stroma, the same compartment in which NADPH and ATP are produced!
Section 14.3 Calvin Cycle
As shown in Figure 14.2, the Calvin cycle takes CO2 and converts it to G3P, a triose phosphate. By comparing their chemical structures, we can observe that G3P is larger and more complex than CO2, which is a good indicator that we are looking at an anabolic pathway. We can confirm this by looking at the coupled reactions, which show NADPH and ATP as inputs that provide energy so the reactions can occur.
The Calvin cycle is a multi-step set of reactions where the starting molecule and ending molecule are the same. Our focus will be on the initial steps of the cycle. As shown in Figure 14.8, the first step of the Calvin cycle is carbon fixation, in which CO2 is “fixed” or bound to a five-carbon molecule called RuBP. This reaction converts inorganic carbon in the form of CO2 gas into an organic molecule. The enzyme that catalyzes this essential reaction is called RuBisCO, and is named for its substrates. This enzyme is very abundant in leaf cells, not only because it has an essential role in photosynthesis, but also because it is very slow in catalyzing the reaction, so plants need a lot of the enzyme to ensure enough product is made.
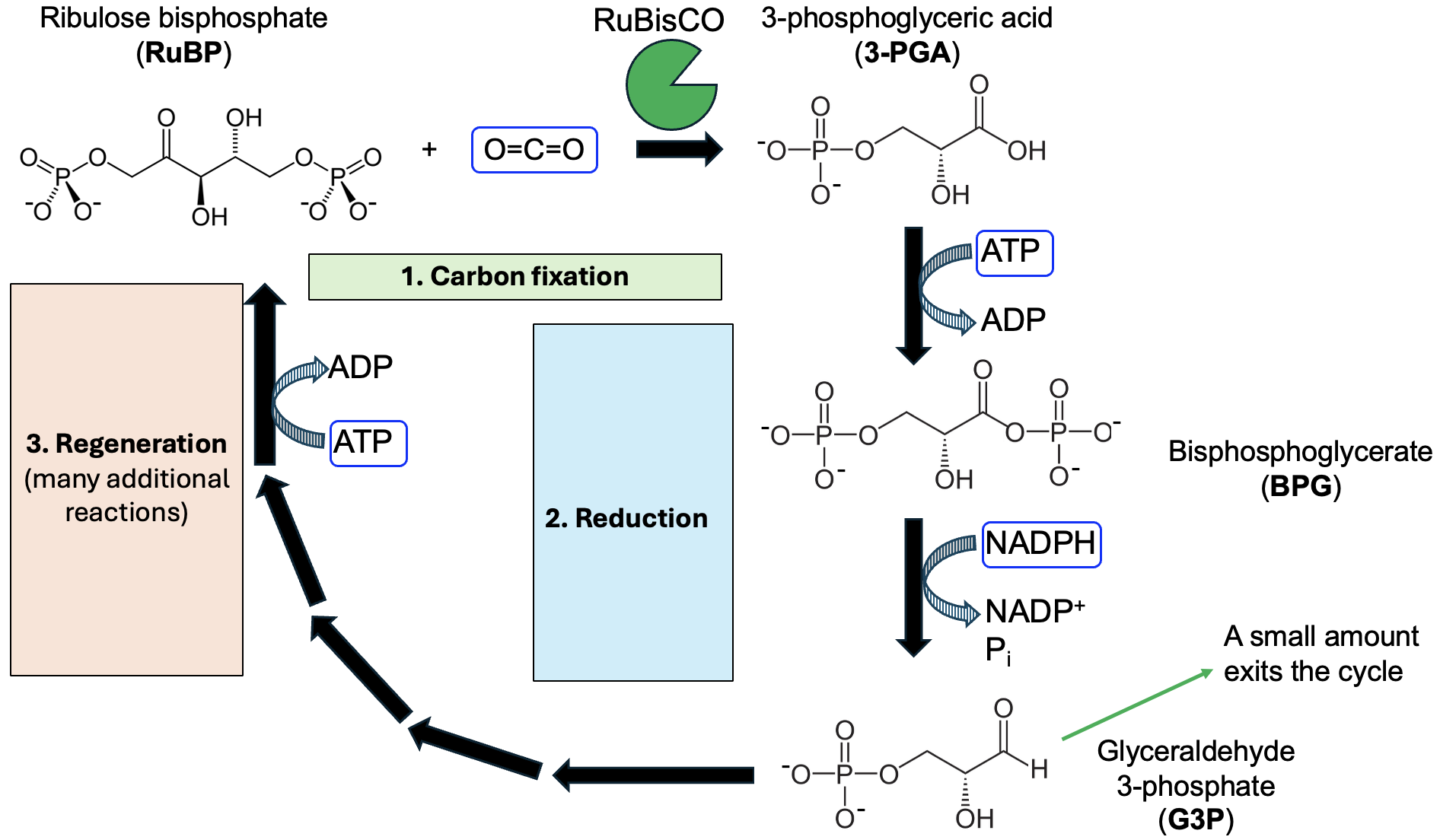
The reaction catalyzed by RuBisCO produces an unstable six-carbon molecule that immediately breaks down into two molecules of 3-PGA, each with three carbons and one phosphate. In the next reaction, we see the first input from the light reactions: 3-PGA and ATP react to form BPG and ADP. Notice that a phosphate is transferred from ATP to 3-PGA, so that the BGP product has two phosphates. Next, BPG reacts with NADPH, causing a phosphate to break off and a hydrogen atom added in its place to produce the G3P product, along with NADP+. This is a complicated reaction, but we know that NADPH [latex]\rightarrow[/latex] NADP+ is an oxidation, so BPG is reduced to G3P. This reaction gives this part of the Calvin cycle its name, reduction, as the carbon input is reduced as NADPH from the light reactions is oxidized.
At this point, a small amount of G3P can exit the cycle and be for other processes. However, the majority of the G3P is needed for the remaining steps, which are the bulk of the Calvin cycle. Comparing G3P to RuBP, we can see that this regeneration phase will require energy input to go from three carbons and one phosphate in G3P, to five carbons and two phosphates in RuBP. Indeed, ATP is needed in this phase as well, serving as a source of phosphates and energy to regenerate the RuBP needed for the next turn of the cycle.
As shown in Figure 14.8 and summarized in Figure 14.2, the products of the Calvin cycle are G3P (albeit a small amount of the total G3P generated), as well as ADP, Pi, and NADP+. The possible fates of G3P will be discussed in the next section, while Figure 14.2 shows the fate of the other products—back to the light reactions!
Section 14.4 Metabolic Connections within Plants
Let’s recap what we’ve covered thus far in this chapter: plants capture light energy and use it to make ATP and NADPH in the light reactions, which also produce O2. Then, ATP and NADPH are used to reduce CO2 and build it into G3P, which is a 3-carbon sugar. But then what?
You might not recognize G3P, but we encountered it before, in the reactions of cellular respiration (Chapter 13). Look at steps 6 and 7 of glycolysis, from Figure 13.5, and compare with the key molecules in Figure 14.8. These are the same molecules in the opposite order. The reactions are catalyzed by the same enzymes, but occur in the indicated directions due the concentrations of substrates and products at that time. Figure 14.9 is a summary model of reactions involving glucose., showing that G3P is an intermediate for both the synthesis and oxidation of glucose.

What happens to the G3P produced in photosynthesis depends on the needs of the plant at the time it is produced. Figure 14.10 shows they key metabolic pathways that utilize G3P, along with the key outputs of the chloroplast and mitochondria. At all times, plant cells need ATP for their cellular functions, so some of the G3P produced by the chloroplast is oxidized using cellular respiration in mitochondria. On very cloudy days or during the night, G3P is not produced by photosynthesis, so plants must use their energy reserves to ensure a constant supply of ATP. As we learned in Chapter 3, starch is an energy storage polysaccharide, and catabolic pathways break down the starch into glucose that can be further metabolized by cellular respiration to produce ATP (Figure 14.9 and Figure 14.10).
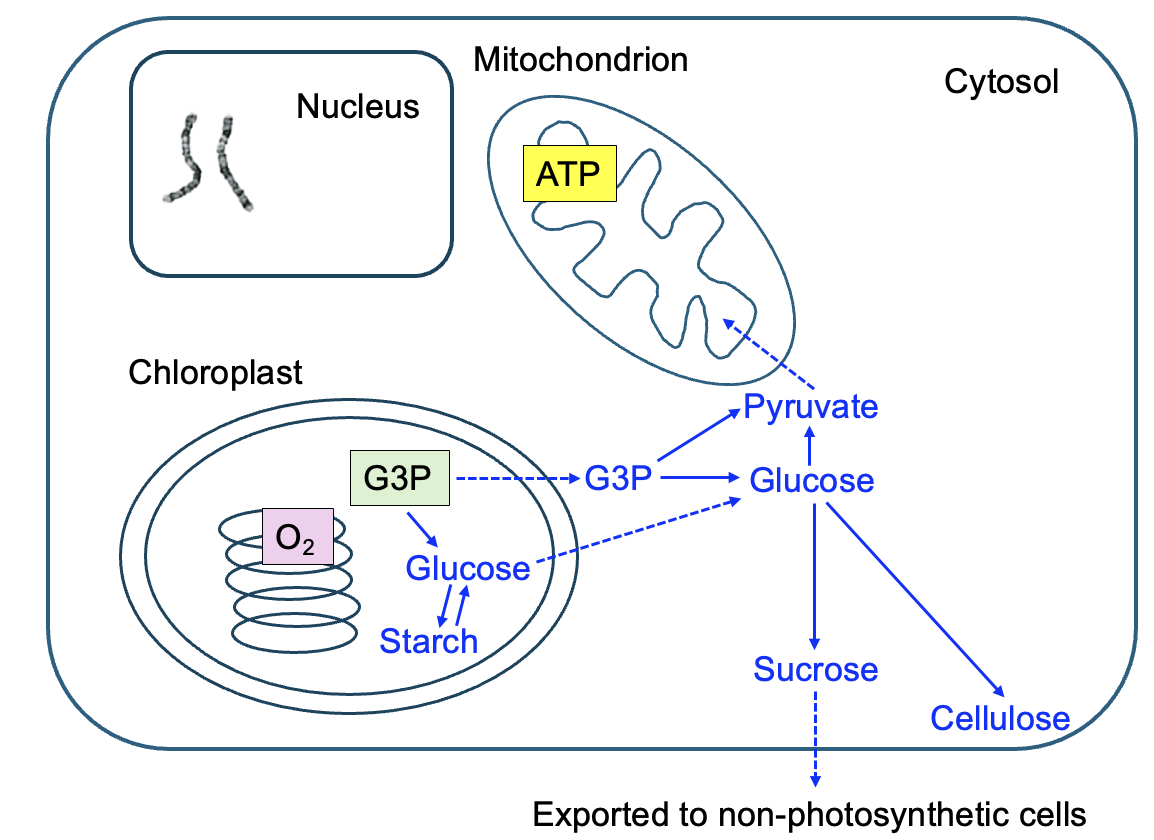
If abundant photosynthesis is occurring, with lots of G3P produced, anabolic pathways are favored. Within the chloroplast, G3P can be combined to make glucose, and glucose molecules combined to produce starch. G3P that is exported from the chloroplast into the cytosol is built up into glucose and then used to create macromolecules like cellulose. Recall from Chapter 3 that cellulose is a polysaccharide of glucose that provides structure support in the plant cell wall. Glucose can also be combined with another sugar, fructose, to make the disaccharide sucrose. Sucrose is easily transported within the plant to share with other plant cells that cannot perform photosynthesis, like root cells. Overall, if a plant is growing, this indicates that photosynthesis is producing more G3P than the plant needs for maintaining cellular functions, allowing more anabolic pathway reactions to occur.
Figure 14.11 is a simplified version of the metabolic transit map in Figure 12.1, and reiterates a key point from that map: metabolic pathways are integrated such that molecules from one pathway can feed into other pathways. Not only can the G3P output of photosynthesis be used to produce polysaccharides, but the intermediates of cellular respiration can be transferred to make other types of molecules.
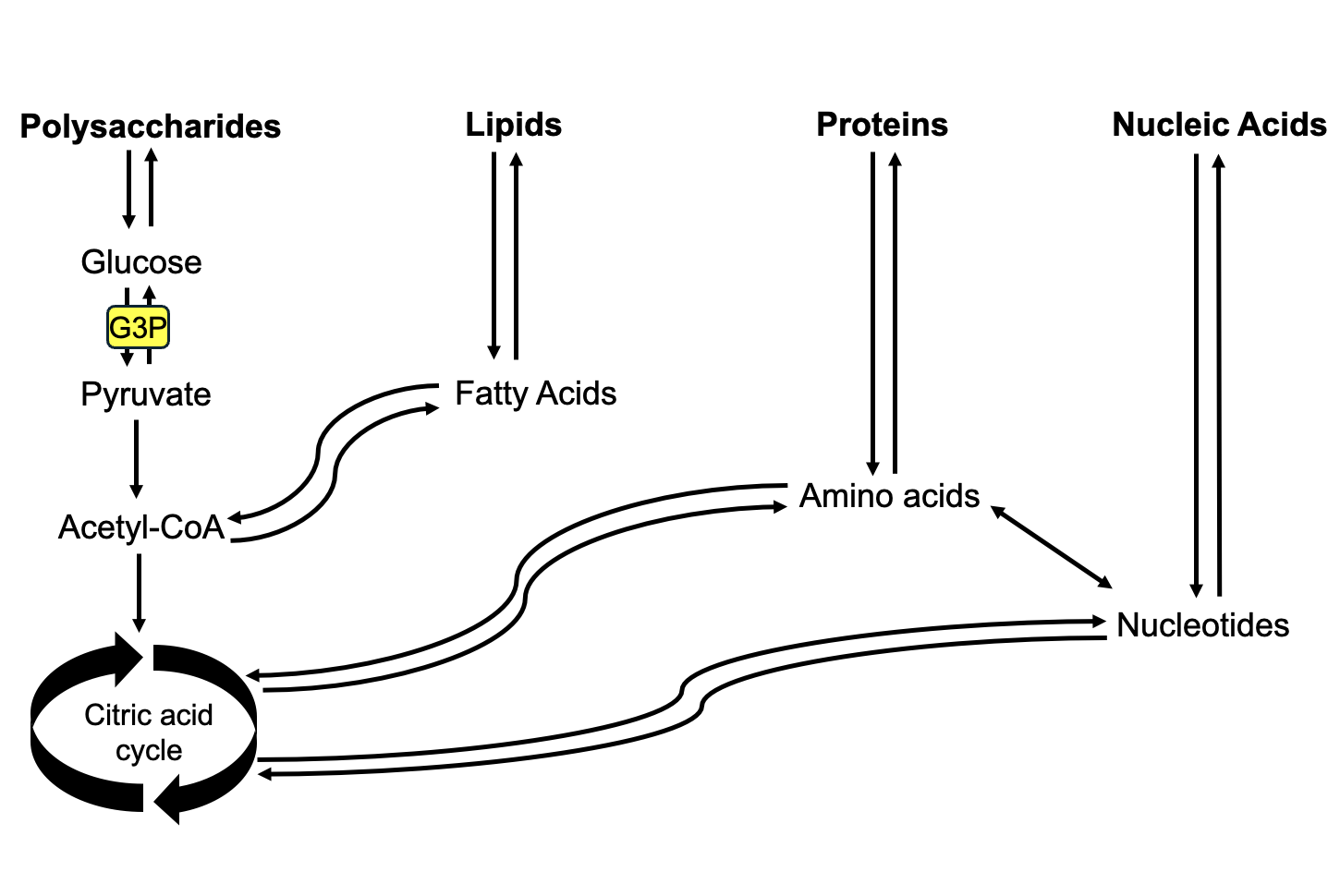
For example, the acetyl-CoA produced by pyruvate oxidation is an intermediate building block for fatty acids. Instead of being further oxidized in cellular respiration, it can be diverted to make membrane lipids and triacylglycerol, a more concentrated energy storage molecule. Ultimately, all the macromolecules needed by plants are produced through modification of the G3P output of photosynthesis, which started as CO2 from the air.

