13 Cellular Respiration
Learning Objectives
- Describe the principles of cellular respiration, including the transformations of energy and matter that occur in the process
- Explain why respiration generates more ATP than fermentation, and why cells produce more ATP from the metabolism of fats compared to the metabolism of sugars
- Use a model of cellular respiration to explain how changes to metabolic substrate or product concentrations in one part of the process affect other parts of the process
- Use a model of cellular respiration to predict how cellular respiration is affected by mutations in genes encoding cellular respiration proteins or by chemicals
Now that we have explored cellular forms of energy (Chapter 10), energetic coupling (Chapters 10–12), and major types of reactions in metabolism (Chapter 12), we can apply that understanding to major to two metabolic pathways: cellular respiration (this chapter) and photosynthesis (Chapter 14). Cellular respiration is the major catabolic pathway that generates ATP for cells, which is required for all synthesis reactions, primary active transport, and many other cellular functions.
Before delving into the details, let’s first revisit the big picture (Figure 13.1). The oxidation of glucose to CO2 and H2O is very energetically favorable (ΔG =-2800 kJ/mol) (Figure 13.1a).
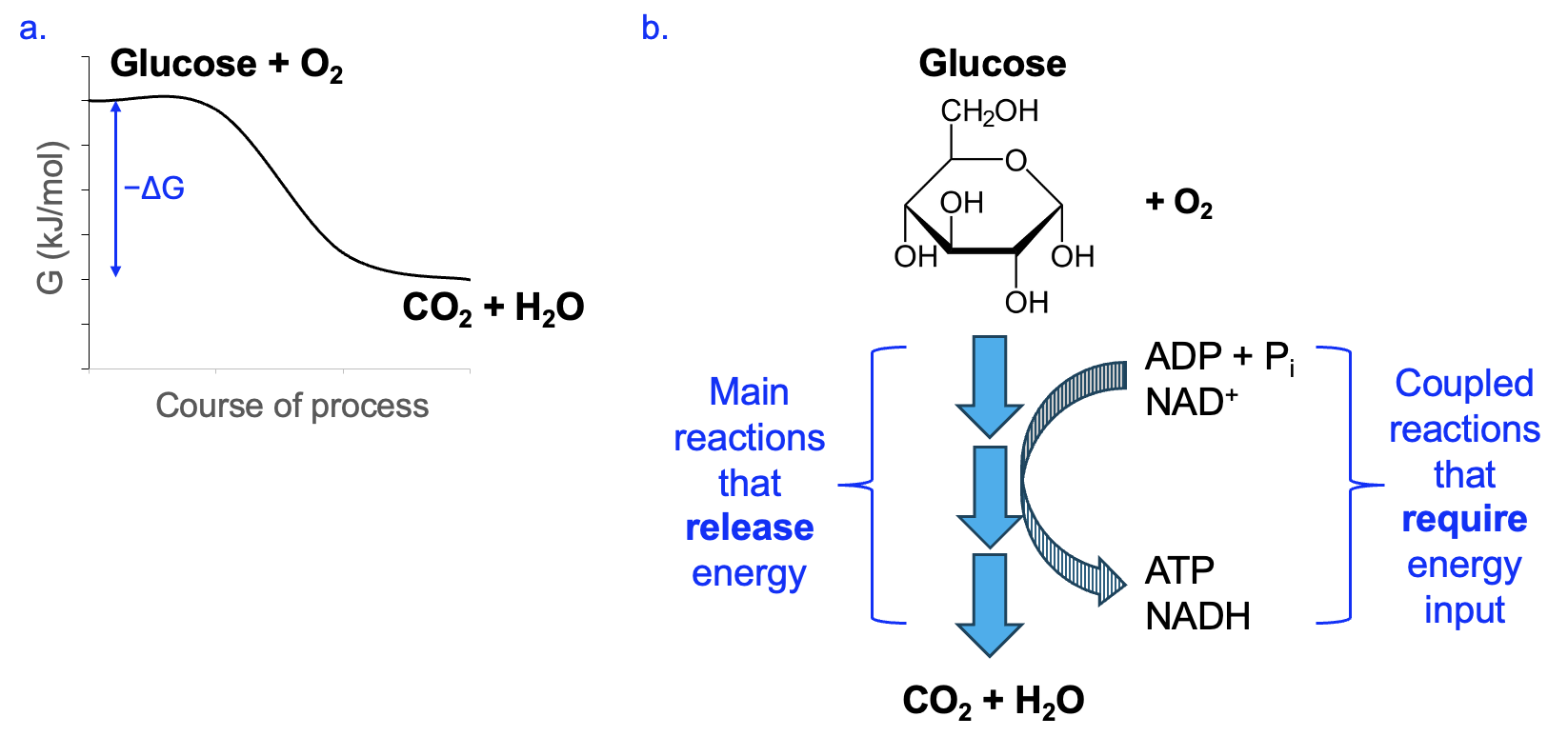
However, if the entire reaction were to happen as one step, a large activation energy would be required, and a huge release of energy, mostly in the form of heat, would follow. Neither of these things is very compatible with normal cellular functions, and so this does not happen in cells. Instead, cellular respiration utilizes many reactions organized in a pathway. These reactions are catalyzed by enzymes to reduce the activation energy and allow cells to capture a significant amount of the energy released in the form of energy management molecules, ATP and NADH, in coupled reactions (Figure 13.1b).
Interestingly, as we will see as we go through these pathways, please note that the glucose and O2 reactants from the summary equation never react with each other! Likewise, the CO2 and H2O products are each made at different stages of the process. Thus, our summary equation shows the big picture but does not represent how the process works in cells.
Chapter Outline
Section 13.1 Overview of Carbon Oxidation
Section 13.2 Glucose Oxidation Reactions
Section 13.3 Electron Transport and Oxidative Phosphorylation
Section 13.4 Connections Between Phases
Section 13.1 Overview of Carbon Oxidation
As you will see shortly, cellular respiration is a complex metabolic pathway with many stages and individual reactions, and it is easy to lose sight of the overall picture. To help organize your understanding of cellular respiration, think of this process as occurring in two phases: carbon oxidation, followed by electron transport and oxidative phosphorylation.
In the carbon oxidation phase, food molecules (this could be sugars like glucose, or fatty acids) are fully oxidized to CO2 in a series of reactions (Figure 13.2).
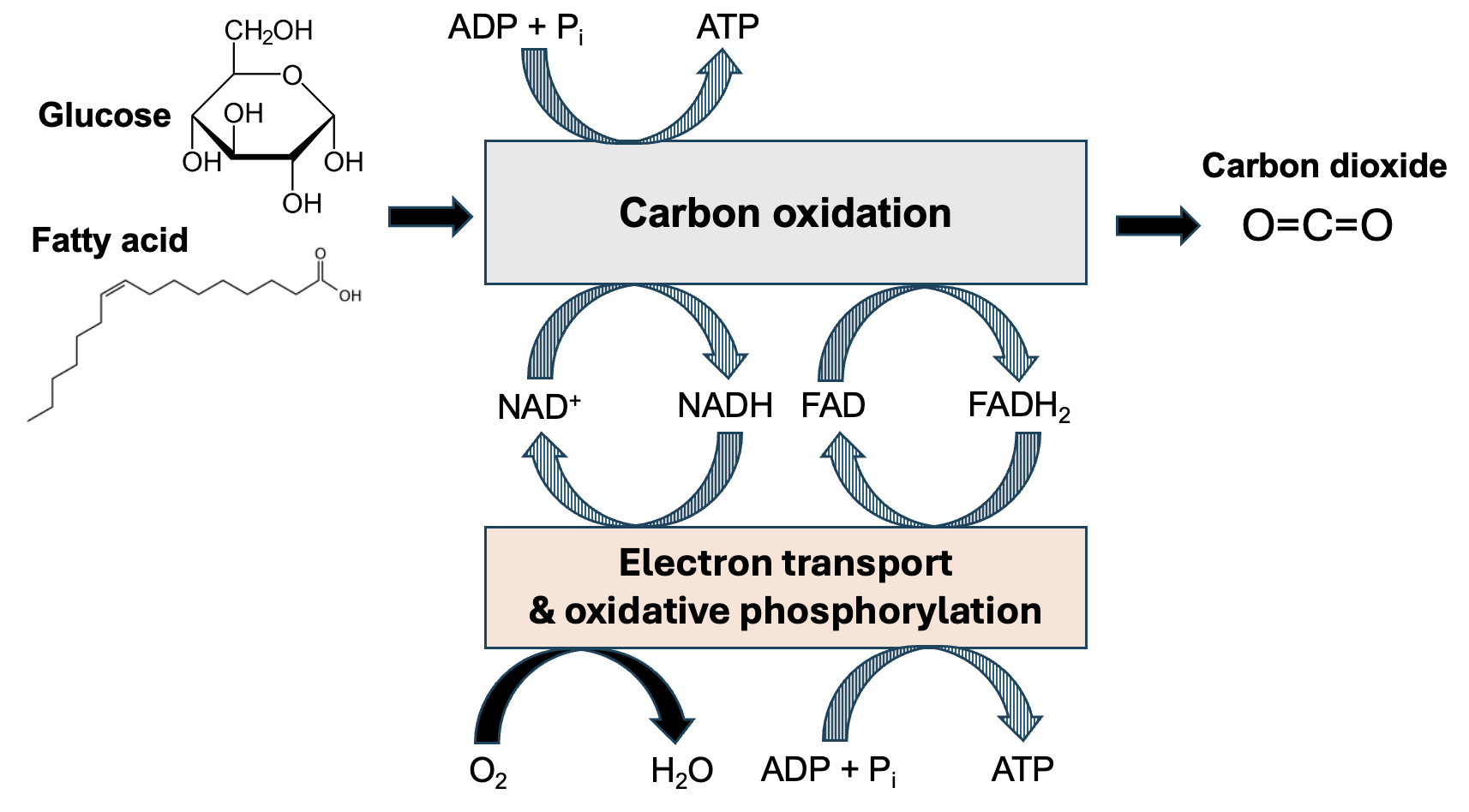
Some energy released in these reactions is coupled directly to the formation of a small amount of ATP. However, remember that in redox reactions, oxidation is paired with reduction. So as the carbon in these food molecules is oxidized, energy management molecules are reduced, capturing more of the energy released. NAD+ and another electron carrier, FAD, are reduced to NADH and FADH2, respectively. A small amount of energy released by this phase is captured by the formation of ATP. However, much more of the energy released from the breakdown of food molecules is captured by the formation of NADH and FADH2. In section 13.3 of this chapter, we will see how additional reactants with NADH and FADH2 are used to generate more ATP.
In eukaryotic cells, mitochondria are the sites of many cellular respiration reactions. Figure 13.3 shows the general structure of mitochondria.
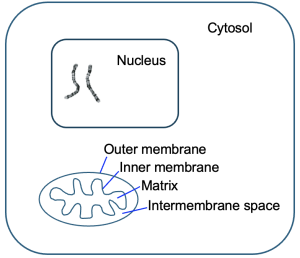
Remember from Chapter 9 that mitochondria have both outer and inner membranes. Each of these membranes creates an internal compartment. The intermembrane space is the compartment between the mitochondrial membranes, and the matrix is the compartment within the inner membrane. We shall see that both compartments and the inner mitochondrial membrane are important for the reactions of cellular respiration. Notice the wavy structure of the inner mitochondrial membrane—this is to maximize the surface area of this membrane to support the reactions occurring there. In bacteria, which lack organelles like mitochondria, similar reactions still occur, but in the cytosol and across the plasma membrane.
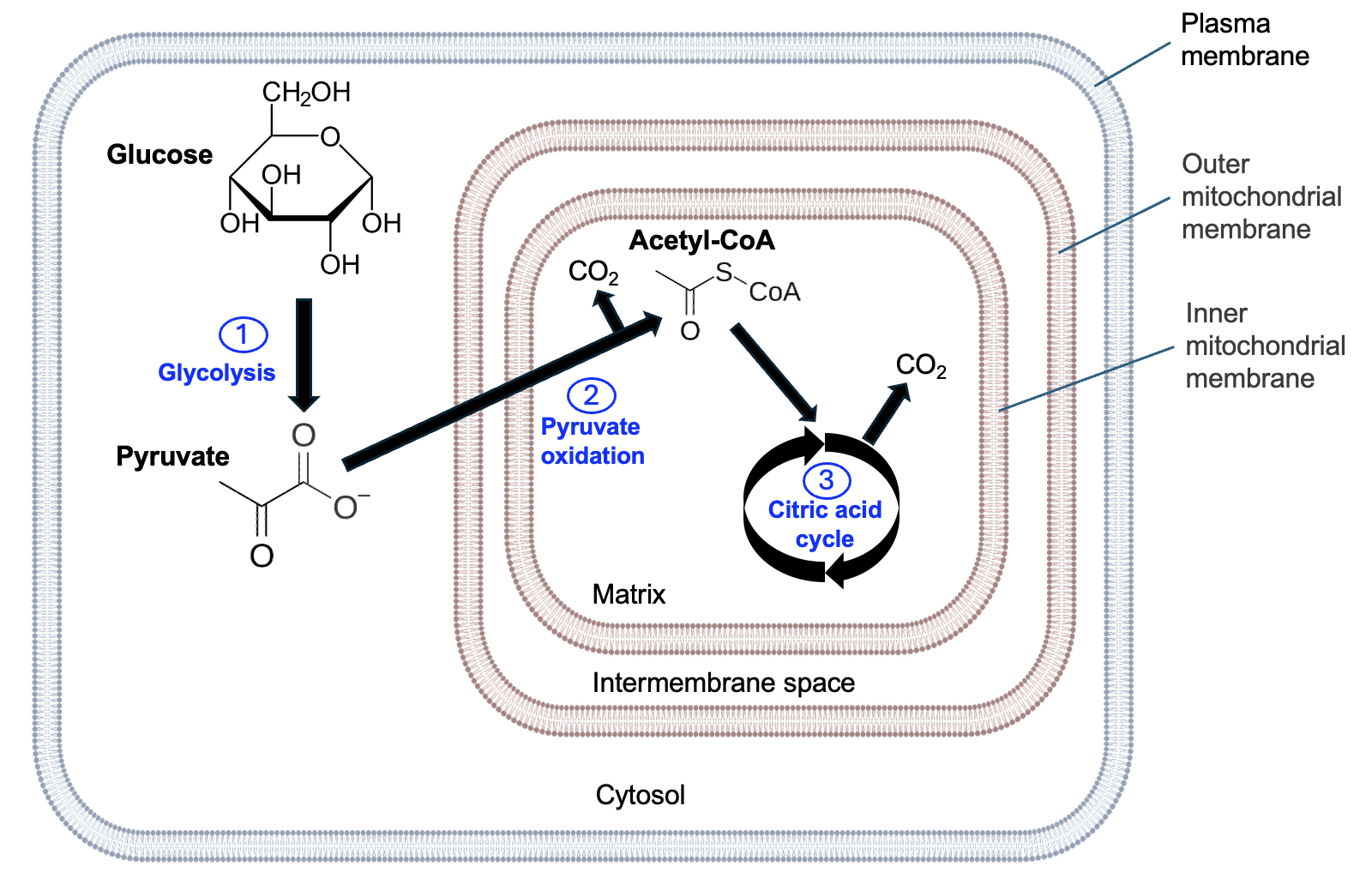
Glucose is a common energy source for many cells, so much of this chapter will focus on the steps cells use to harvest energy from glucose. The oxidation of glucose to CO2 occurs in three stages: glycolysis, pyruvate oxidation, and the citric acid cycle (which also known as the Krebs cycle). Figure 13.4 shows the cellular locations and the main carbon outputs of each of these steps. Stage 1, glycolysis, occurs in the cytosol and produces pyruvate. The pyruvate is transported through both mitochondrial membranes, and, by the process of pyruvate oxidation, is converted to acetyl-CoA and CO2 is released. Lastly, acetyl-CoA is further oxidized by the citric acid cycle, releasing more CO2.
While we won’t go into the details, remember that fatty acids can also be metabolized. A set of reactions occurring in the matrix, called beta oxidation, converts fatty acids into molecules of acetyl-CoA. Acetyl-CoA enters the citric acid cycle, and the remaining steps are the same for both glucose and fatty acids.
Section 13.2 Glucose Oxidation Reactions
The remainder of this chapter will focus on glucose oxidation. Please note, while the full reactions (and in some cases, relevant enzymes) are shown, your focus should be on the net inputs and outputs of each stage, recognizing the specific coupled reactions occurring, and explaining the overall energetics of each process. Use the net inputs and outputs to track the carbon and energy transformations and identify links among processes. This will help you develop a model that can be used to make predictions of how a change in one part of the process could impact other parts of the process.
Glycolysis
The first stage of glucose oxidation is glycolysis, or the splitting of sugar. Figure 13.5 shows the ten reactions of glycolysis, which begins with a molecule of glucose, a sugar with six carbons. Notice that in reaction 4, the molecule is split into two molecules, each with three carbons. Steps 6–10 occur in duplicate, and the final reaction of glycolysis produces two molecules of pyruvate, each with three carbons.
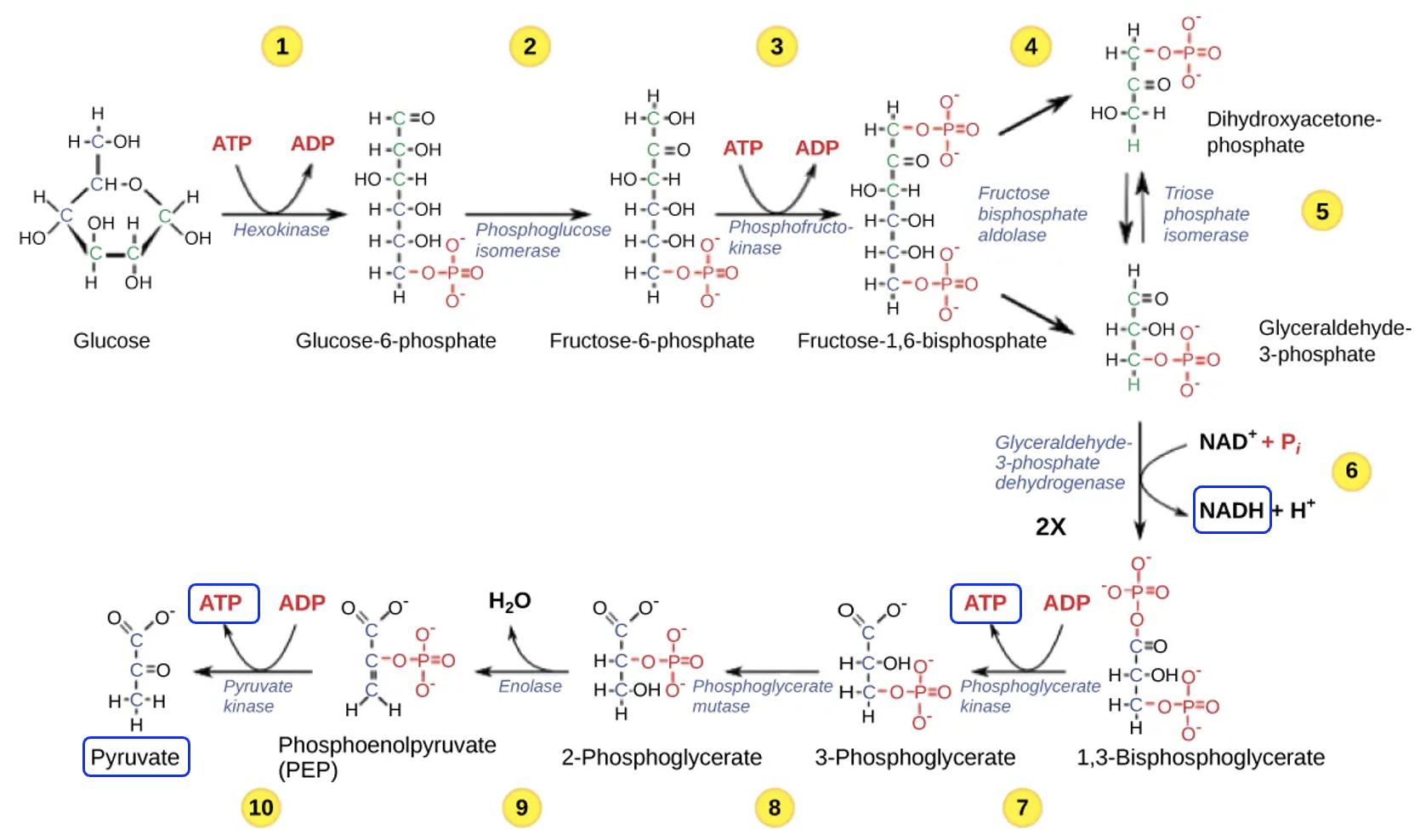
As glucose is transformed into two molecules of pyruvate, energetic coupling also occurs. In reactions 1 and 3, ATP is an input (reactant), while in reactions 7 and 10, ATP is an output (product). Since steps 7 and 10 occur in duplicate, four ATP are produced, which means two net ATP per glucose molecule are formed. In step 6, assuming there is sufficient NAD+ reactant, coupled reactions produce two NADH. Because NAD+ gains electrons and is therefore being reduced to NADH, we know that in the main reaction, the glycolysis intermediate, glyceraldehyde-3-phosphate (abbreviated G3P) is being oxidized to 1,3-bisphosphoglycerate or BPG.
Overall, no carbon is lost in glycolysis, but the carbon atoms have been rearranged in such a way to allow NADH and ATP to be produced. Some of the energy released as glucose is broken down is captured in the formation of NADH and ATP and some energy is lost as heat. The pyruvate produced from glucose breakdown will go on to the next stage for further breakdown. Because the formation of ATP and NADH requires energy input, and these reactions are coupled to reactions in glycolysis, we know that glycolysis is catabolic and energetically favorable. What is the purpose of the ATP in steps 1 and 3? Well, even though the pathway is energetically favorable, glucose is a stable molecule. The addition of two phosphates in the earlier steps help to destabilize glucose and make it more reactive.
Pyruvate Oxidation
Glycolysis occurs in the cytosol of the cell, but the pyruvate product is then transported into the matrix of the mitochondria, passing through both the outer and inner mitochondrial membranes. In the complicated reaction shown in Figure 13.6, a carboxyl group is lost from pyruvate. This reduces the number of carbons from three to two as a molecule of CO2 is released. Assuming there is sufficient NAD+ reactant, oxidation of pyruvate allows NAD+ to be reduced to NADH in a coupled reaction. Finally, coenzyme A (CoA) is added, resulting in the two-carbon acetyl-CoA molecule. We won’t worry too much about CoA, as it will be removed at the start of the next phase, which is the citric acid cycle.
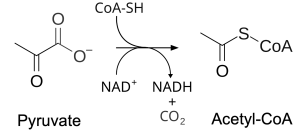
Overall, in this stage, CO2 is lost from each pyruvate, corresponding to two carbons of the original glucose molecule. Some energy is transferred to NAD+ to produce NADH and some is lost as heat. The acetyl-CoA product of the reaction will be broken down further in the next phase.
Citric Acid Cycle
The third and final stage of glucose oxidation is the citric acid cycle, also known as the Krebs cycle, which also occurs in the matrix (Figure 13.7). The input to this process is acetyl-CoA that was produced in pyruvate oxidation. In the first chemical reaction of the citric acid cycle, this two-carbon acetyl-CoA reacts with a four-carbon molecule called oxaloacetate to form citrate, a six-carbon molecule for which the citric acid cycle is named. Figure 13.7 shows all eight reactions of the citric acid cycle but not the enzymes that catalyze each step. Notice that the final reaction of the cycle produces oxaloacetate, which can react with the next acetyl-CoA molecule.
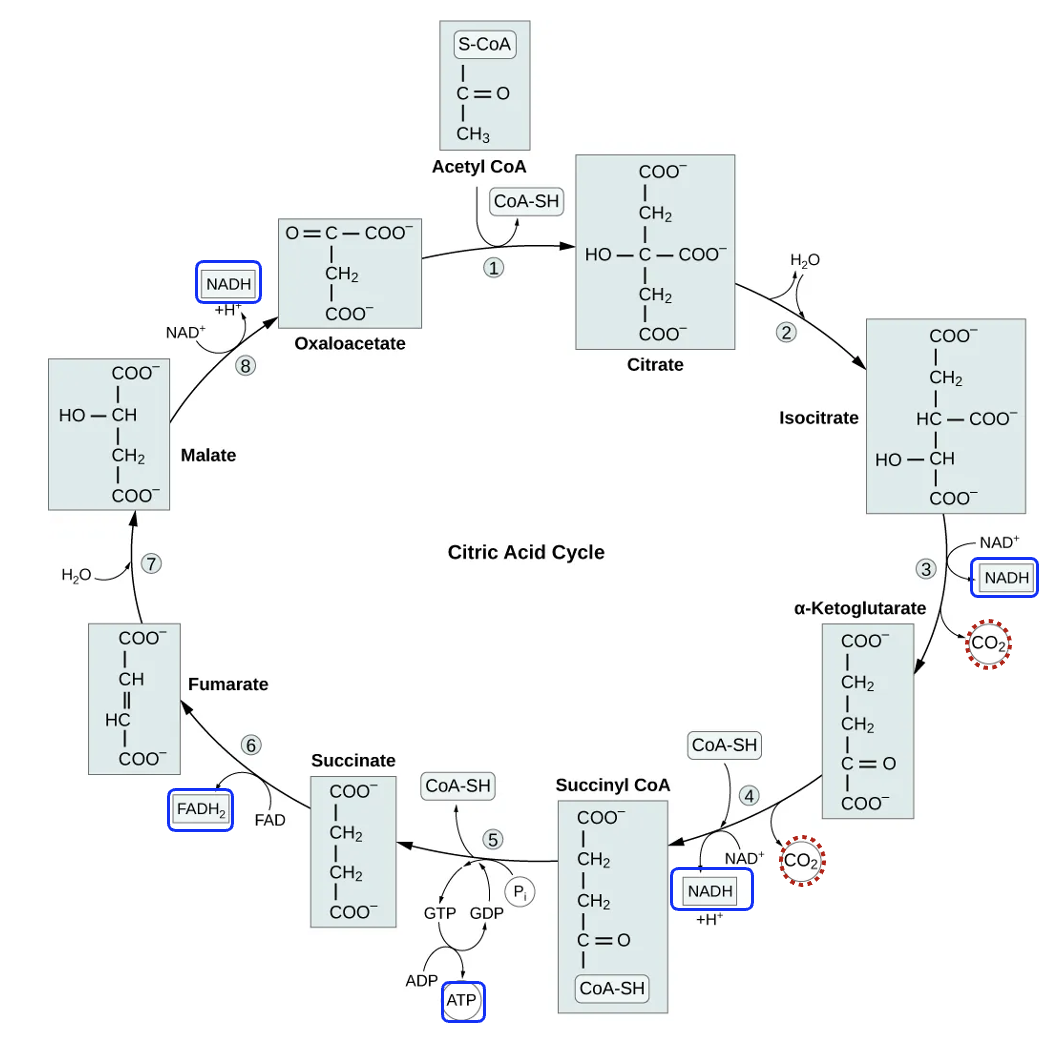
Focusing first on the carbon, notice that steps 3 and 4 have CO2 as a product. Each acetyl-CoA molecule contributed two carbons to the cycle, so by step 4 all the carbon added to the cycle (and therefore all the carbon originally in glucose) has been transformed into CO2. What happens to the CO2 from this stage and pyruvate oxidation? Well, being a gas, it can use simple diffusion to cross cellular membranes. In our cells, the released CO2 diffuses into the blood and transported to the lungs, where CO2 can diffuse into the air.
In the citric acid cycle, steps 3 and 4, along with steps 5, 6, and 8, are all coupled to the production of energy management molecules. In three different reactions NAD+ is reduced to NADH, indicating that the main reaction molecule is oxidized. Reaction 6 is coupled to a different energy management molecule FADH2. FAD reactant is converted to FADH2 product, which is a gain of two hydrogens, or a reduction. Can you identify the carbons in succinate that were oxidized to make the product fumerate? The citric acid cycle also produces one molecule of ATP per acetyl-CoA, but most of the energy released from the breakdown of acetyl-CoA was coupled to the formation of NADH and FADH2, or was lost as heat. Overall, since reactions coupled to the oxidation of acetyl-CoA produce ATP, NADH, and FADH2, this is an energetically favorable, catabolic pathway.
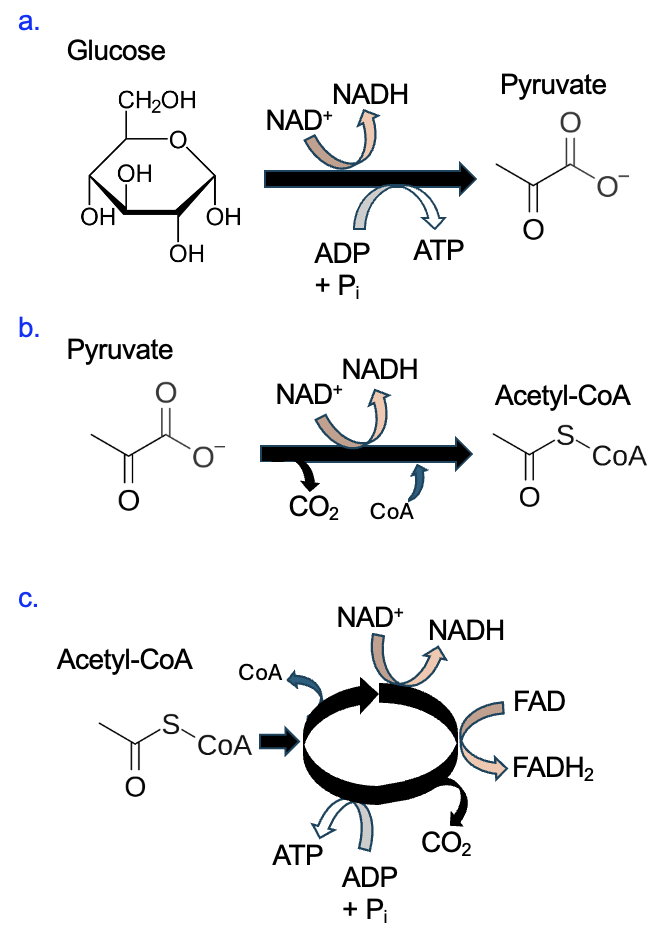
To summarize the entire process, energetically favorable glucose oxidation reactions convert all carbon in the glucose molecule to CO2. Some energy released by breaking down glucose is captured by producing ATP, but the majority is used to make NADH and FADH2, and some is lost as heat. Figure 13.8 summarizes the net inputs and outputs for each stage of glucose oxidation.
Section 13.3 Electron Transport and Oxidative Phosphorylation
The second major phase of cellular respiration is electron transport and oxidative phosphorylation. In this phase, electrons from NADH and FADH2 are used to reduce O2 to H2O. The energy released from this process is indirectly coupled to the synthesis of ATP. This process produces most of the ATP for the cell.
Figure 13.9 shows the proteins relevant for this process, located in the inner mitochondrial membrane. Complexes I, II, III, and IV are electron transport chain proteins that use redox reactions to transfer electrons.
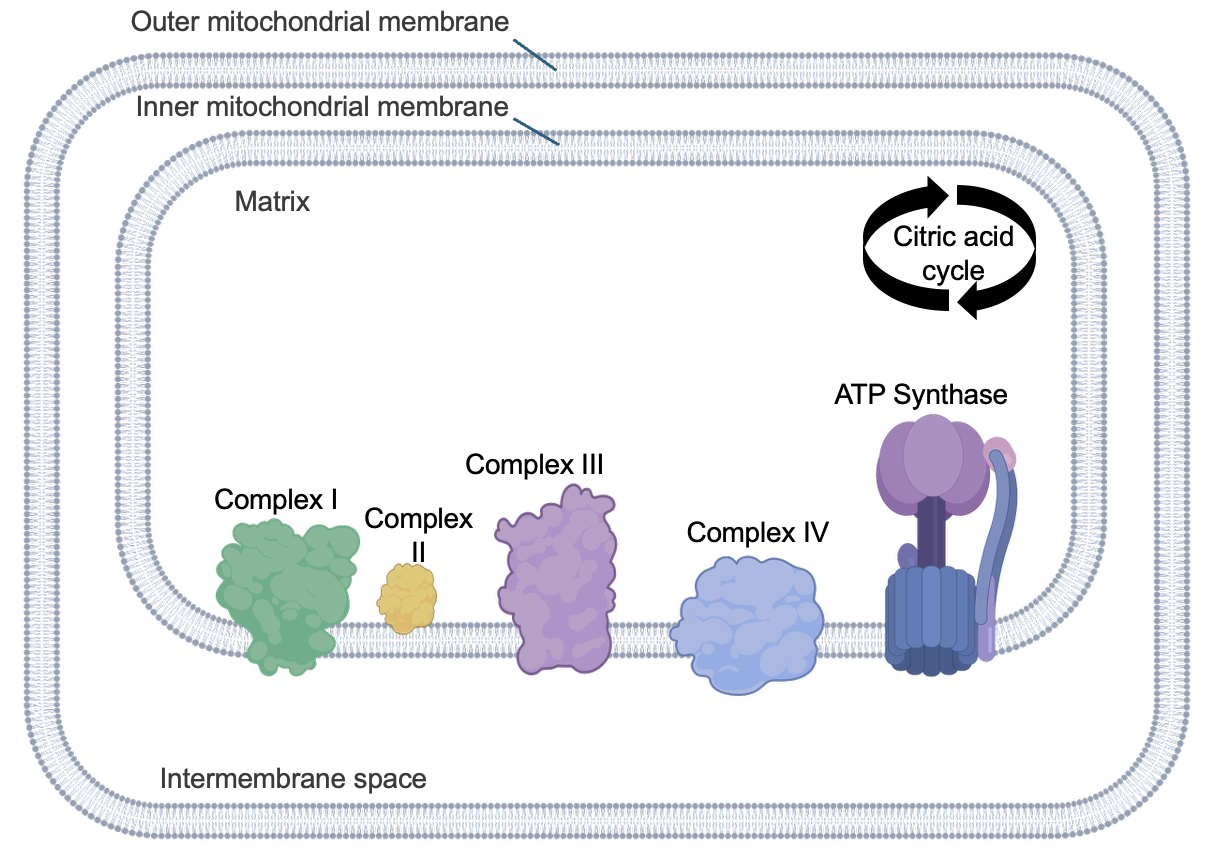
There are other small molecules that assist with transferring electrons that are not shown in Figure 13.9. Another key protein is ATP synthase, which is also embedded in the inner mitochondrial membrane. As its name implies, this protein is responsible for the synthesis of ATP.
To understand how this process works, let’s start with the electron transport chain and focus on the fate of electrons associated with NADH (Figure 13.10). The NADH input is converted to NAD+ by Complex I. Because NADH is losing electrons, this an oxidation, and it is coupled to the reduction of Complex I. Complex I is then oxidized and its electrons are used to reduce Complex III. (While not shown in Figure 13.10, FADH2 is oxidized by a different mechanism and indirectly contributes electrons to Complex III.) Since each complex in the chain has a slightly higher affinity for electrons than the previous one, electron transfers between complexes are energetically favorable, which is why electrons move through the electron transport chain.
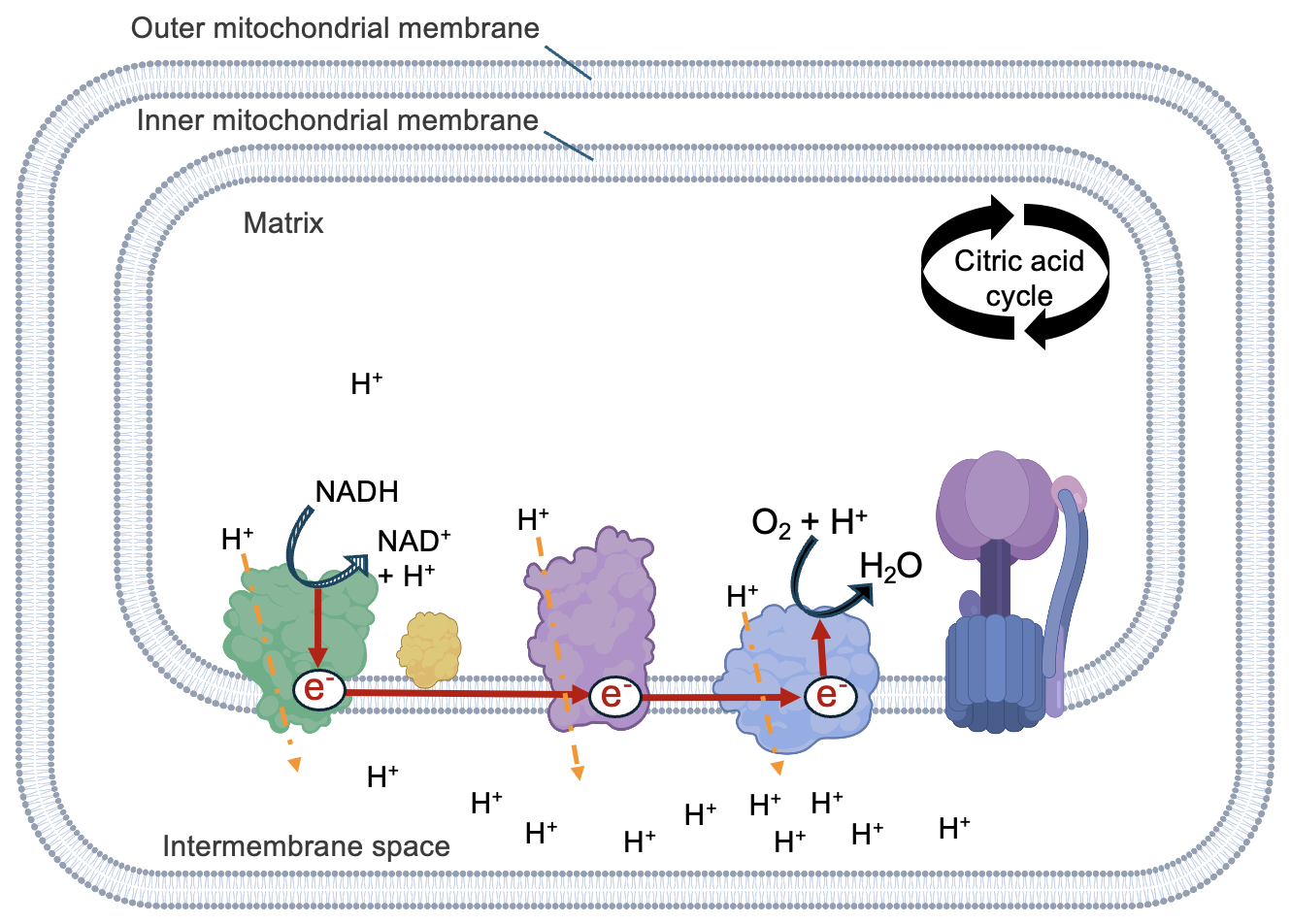
The redox reactions continue until electrons reach the final electron acceptor, O2. In a very carefully controlled reaction, O2, 4 electrons, and 4 protons combine in the matrix to make two molecules of H2O. This is the only reaction in cellular respiration to directly use O2, and in fact, the only reason why we need to breathe oxygenated air. In the absence of O2, no electron movement occurs and the entire electron transport chain (and proton pumping) halts.
Because O2 has a high affinity for electrons (recall the high electronegativity of oxygen), the passage of electrons from NADH to O2 is energetically favorable. Thus, with each redox reaction, some energy is released that is available to support some unfavorable process. Figure 13.10 shows that some complexes transport protons from the matrix to the intermembrane space, coupled to the redox reactions of electron transport. The transport of protons forms a concentration gradient across the inner mitochondria membrane, with the higher proton concentration in the intermembrane space. The formation of water in the matrix further contributes to the gradient as it decreases the concentration of protons there.
Because the protons are moving in an energetically unfavorable direction, from an area of low to high proton concentration, the electron transport chain complexes are also referred to as proton pumps. Overall, the favorable movement of electrons from NADH to O2 is coupled to the unfavorable pumping of protons into the intermembrane space, creating non-equilibrium proton concentrations across the inner membrane. Just as we saw in Chapter 11, these non-equilibrium conditions are a source of energy to support an energetically unfavorable process: the synthesis of ATP.
To return to equilibrium conditions, protons need to flow from the intermembrane space to the matrix. Yet, due to their charge, protons cannot cross a functional membrane by simple diffusion. However, part of the ATP synthase protein is permeable to protons, allowing their flow back into the matrix (Figure 13.11).

This energetically favorable movement of protons, from high concentration to low, through ATP synthase is used to power the energetically unfavorable ATP synthesis reaction, using Pi to phosphorylate, or add a phosphate to, ADP. Thus, there are multiple energetically coupled processes to produce ATP: movement of electrons to create a proton gradient, and movement of proteins to create ATP. The ATP produced in the second stage is most of the cell’s usable energy, utilized for anabolic synthesis reactions, primary active transport, transcription, and many other purposes.
ATP Synthase is a complex molecule machine, so we will just briefly touch on its components and operation. Figure 13.12 shows the two main parts of ATP Synthase, which are two motors. The FO motor is embedded in the inner mitochondrial membrane, and contains an opening for protons accessible from the intermembrane space. Protons move through the F0 motor to an exit on the matrix side. This movement of charged molecules causes the motor to spin. This is a transformation of potential energy, in the form of a concentration gradient, into kinetic energy, energy of movement.
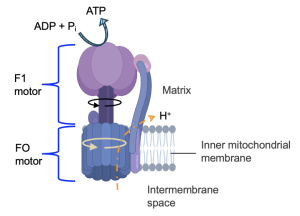
The movement of the FO motor causes the central shaft of the F1 motor to move. The upper part of the F1 motor does not rotate, but the rotation of the central shaft causes several of the upper subunits to change their conformations. Some of these subunits bind to ADP and Pi, and the repositioning of these subunits brings the reactants together to form the ATP product. Thus, this kinetic energy from the rotation of ATP synthase is coupled to the unfavorable production to ATP from ADP and Pi.
To learn more about ATP synthase and watch this process in action, visit this animation:
Section 13.4 Connections Between Phases
Now that we’ve explored the details of each step, revisit the overview in Figure 13.2. From this model, we can observe that the inputs (reactants) for electron transport and oxidative phosphorylation, NADH and FADH2, are from carbon oxidation reactions. So clearly the second phase depends on the first phase. But does electron transport and oxidative phosphorylation contribute to carbon oxidation? According to Figure 13.2, yes! We can observe that as NADH and FADH2 are oxidized by the electron transport chain, NAD+ and FAD are produced. These are required inputs for carbon oxidation. If the electron transport chain slows down, perhaps due to low O2 levels, the NADH and FADH2 oxidation reactions also slow. As concentrations of NAD+ and FAD decrease, the reactions of carbon oxidation also slow.
You can use the model in Figure 13.2 to make many predictions about cellular respiration, supported by reasoning from the more detailed information in this section. Just keep in mind that reactants must be present for reactions to occur and products to be produced. The absence of a critical reactant, such as O2, can prevent an entire process from occurring. By noting the connections between phases, we can predict that low O2 would reduce carbon oxidation, even though it is not a direct input to any of these reactions.
Section 13.5 Fermentation
O2 is directly required only for electron transport chain reactions, where it serves as the final electron acceptor. However, if O2 levels are low, electron transport is slowed and the proton gradient is not formed, slowing ATP synthesis. While carbon oxidation reactions produce a small amount of ATP, these reactions also slow in the absence of oxygen, as necessary reactants are no longer produced by the electron transport chain. Thus, low O2 results in greatly diminished ATP production, to levels that are not survivable by cells.
Fortunately, there is an alternative set of reactions available to cells with the appropriate enzyme. Muscle cells, for example, express lactate dehydrogenase, an enzyme that converts NADH and pyruvate to lactate and NAD+ (Figure 13.13).
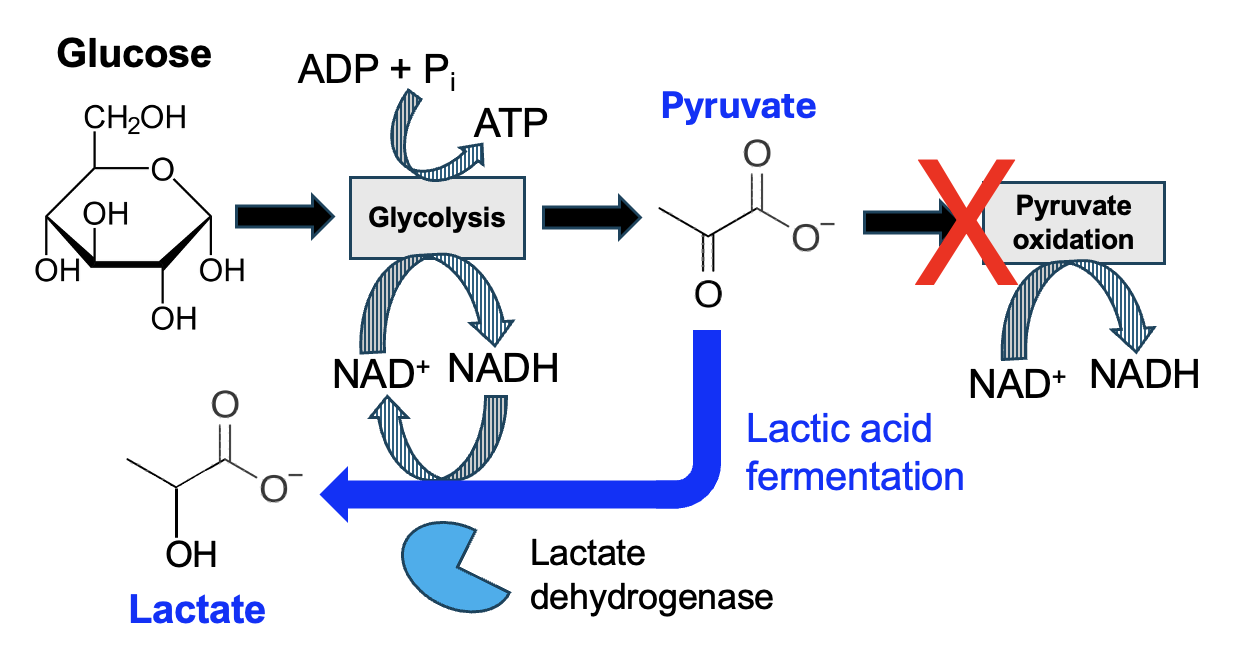
Look carefully at the structures of pyruvate and lactate. Pyruvate has a carbonyl group where lactate has a hydroxyl group, so pyruvate gains hydrogens to be reduced to lactate. This corresponds with the oxidation of NADH to NAD+. As lactic acid fermentation involves the reactions of glycolysis and one additional reaction, fermentation occurs in the cytosol of cells and does not involve the mitochondria (Figure 13.14).
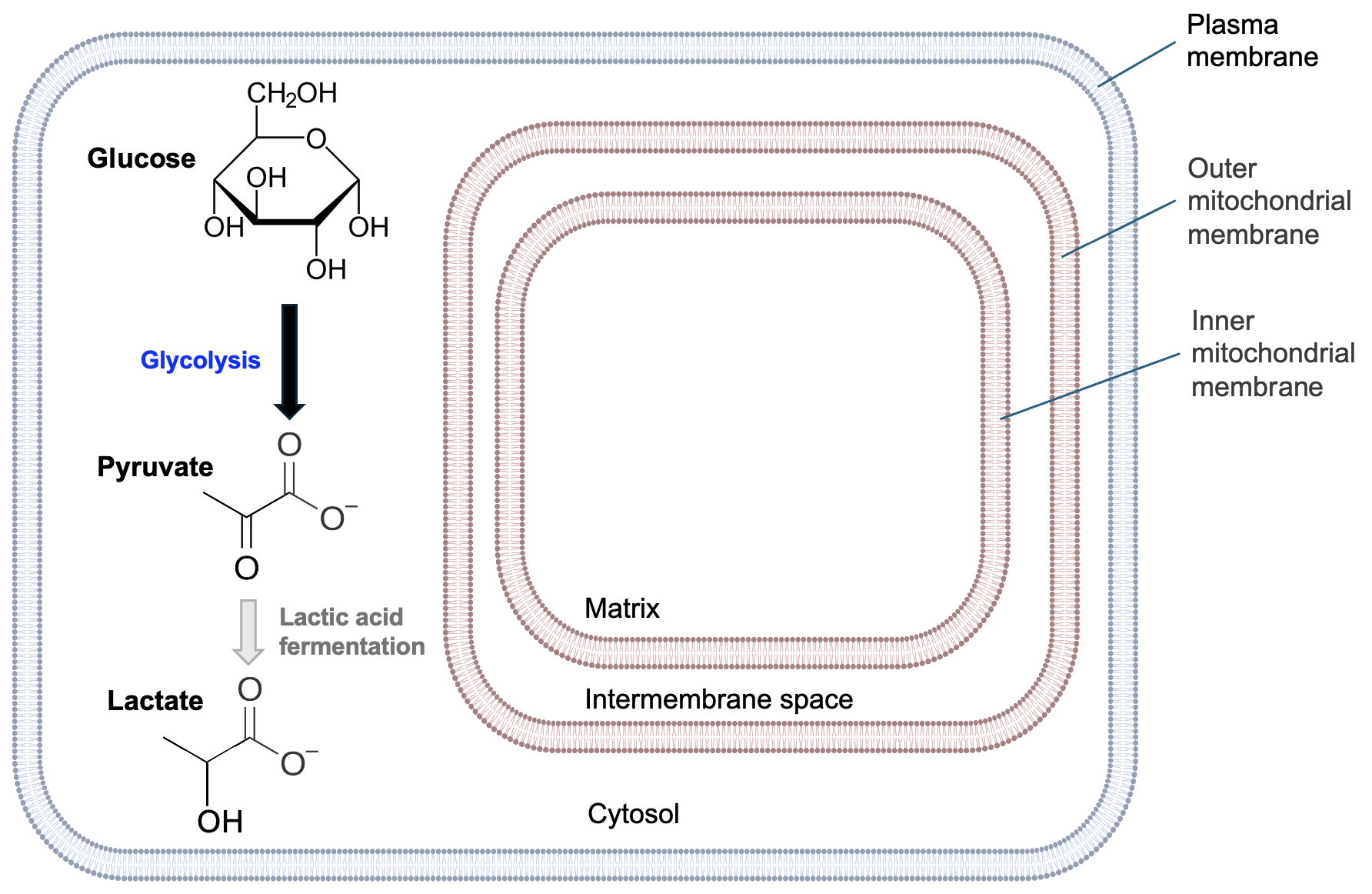
What is the benefit of this reaction? Well, when O2 levels are low, NADH is no longer oxidized to NAD+ by the electron transport chain, effectively decreasing levels of NAD+ as NADH accumulates. Remember that NAD+ is a reactant for many reactions in carbon oxidation, and the reactions that require NAD+ become energetically unfavorable without that reactant. Pyruvate oxidation, for example, no longer occurs with low O2 as NAD+ levels are too low. However, cells with lactate dehydrogenase convert NADH to NAD++. With levels of the key NAD+ reactant increased once again, the reactions glycolysis can proceed, and thus a few ATP can be produced once again. Thus, lactic acid fermentation allows muscle cells to survive a short-term loss of O2.
There are many other types of fermentation reactions, and we will encounter a few more in Chapter 15. These reactions first appeared in bacterial cells under the low O2 conditions of early Earth, long before the emergence of cellular respiration. These reactions don’t necessarily require low O2, but they are a way to produce cellular forms of energy in the absence of O2.
However, if ATP can be produced in the absence of O2, why bother with the complicated process of cellular respiration? Well, while glycolysis and lactic acid fermentation produces two ATP per glucose, much of the free energy of the original glucose molecule remains in lactate. In contrast, cellular respiration fully oxidizes the glucose input to CO2, thus maximizing the available energy. More than 30 ATP are typically produced in full oxidation of glucose, and significantly more ATP are produced by oxidation of fatty acids.

