12 Introduction to Energy Metabolism
Learning Objectives
- Compare and contrast anabolic and catabolic pathways.
- Explain how cells create conditions to couple favorable processes with unfavorable process and why this is required in metabolic pathways.
- Explain how the same enzyme would catalyze a reaction in opposite directions in different cell types or cell compartments
Now that we have established basic energetics principles and applied them to molecule movements, let’s shift our focus to metabolism specifically. Metabolism is all the chemical reactions occurring in the cell. These include the reactions occurring during transcription and translation described in Chapter 4 and Chapter 5, respectively, as well as all the energy metabolism reactions that will be detailed in Chapter 13 and Chapter 14.
Figure 12.1 is representation of metabolism in cells. It is styled like a transit map for a major city’s subway or bus system, showing multiple different routes, spanning different geographic regions, and showing key places where the lines intersect. Metabolism is likewise complex, as there are many different reactions occurring simultaneously in cells. Moreover, like a transit system, there are many different reaction pathways occurring in cells, divided into categories of molecules.
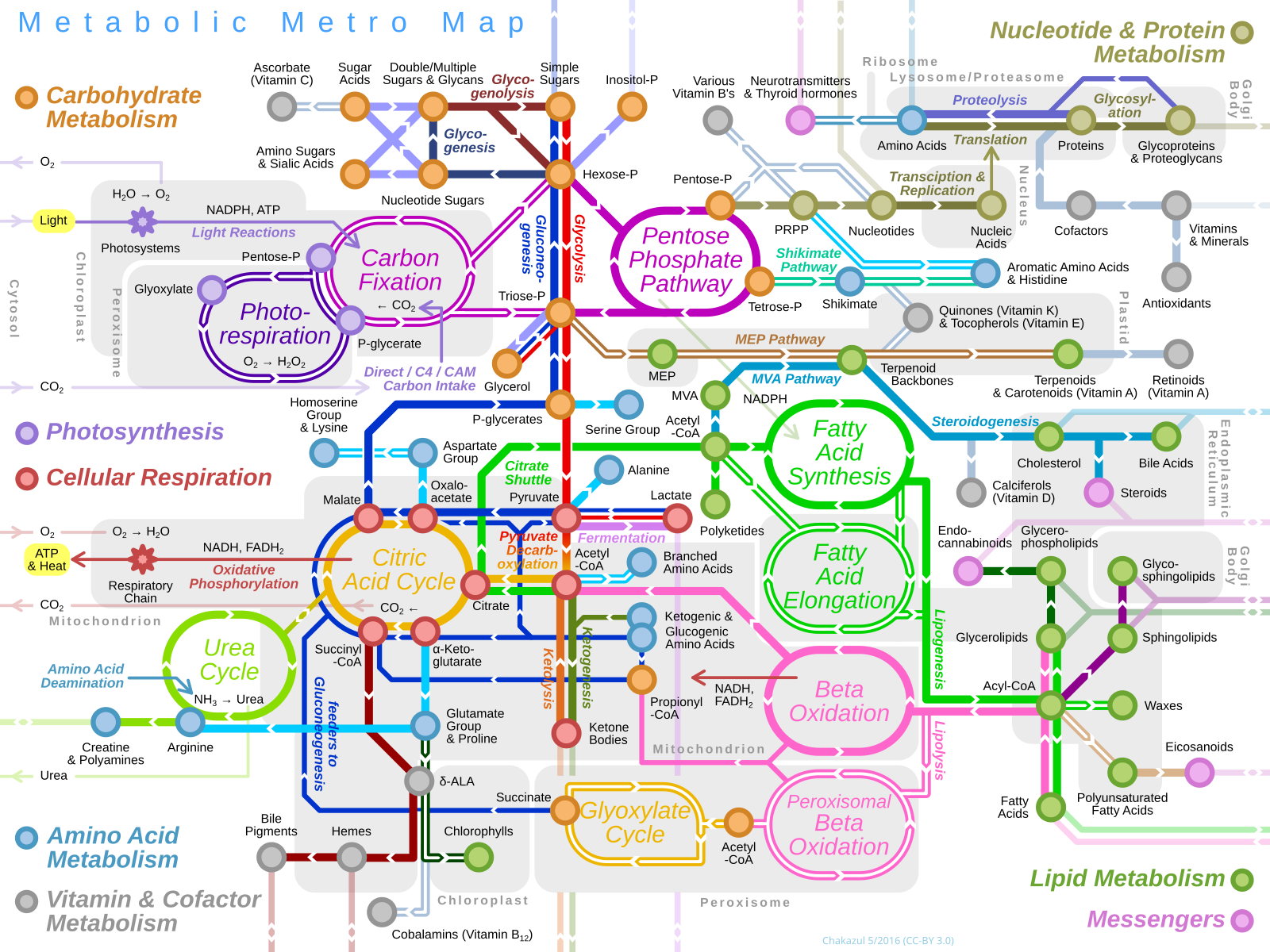
On Figure 12.1, we see some of these categories corresponding to different pathways—Amino Acid Metabolism, Carbohydrate Metabolism, and so on. The filled circles on pathways indicate key molecules produced in those pathways (like station stops), and you may know from experience that if a train is stopped at one station, it can affect progress along the entire route. Similarly, if a key reaction is slowed down, the entire pathway slows as well. Finally, just like some stations connect multiple lines, so too we can see places where one metabolic pathway connects to a different pathway, and molecules can “transfer” from one pathway to another.
We won’t delve too much into the specifics shown in Figure 12.1 beyond exploring cellular respiration and photosynthesis, shown on the left side of the model. However, it’s worth remembering one big idea: in a metabolic pathway, the product of one reaction can become the substrate for another reaction. In so doing, molecules continually move through the pathway, which means continual production of products that cells need.
Chapter Outline
Section 12.1 Anabolic and Catabolic Pathways
Section 12.2 Coupled Reactions with ATP and NAD(P)H
Section 12.3 Reactions Coupled to Anabolic and Catabolic Pathways
Section 12.4 Free Energy Changes in Coupled Reactions
Section 12.5 Metabolic Pathways and Energetic Favorability
Section 12.1 Anabolic and Catabolic Pathways
For the purposes of our discussion, we can set aside the complex network of metabolic pathways and instead focus on two broad categories of metabolic pathways: anabolic and catabolic. Anabolic pathways are synthesis pathways. As shown in Figure 12.2, starting from the bottom left, a series of chemical reactions is used to create more complex molecules from simpler starting molecules. First, CO2 and water are reacted to produce simple sugars like glucose. These are essentially the reactions of photosynthesis and some additional reactions. Then, as we learned in Chapter 3, those glucose monomers can be joined to produce the macromolecule starch.
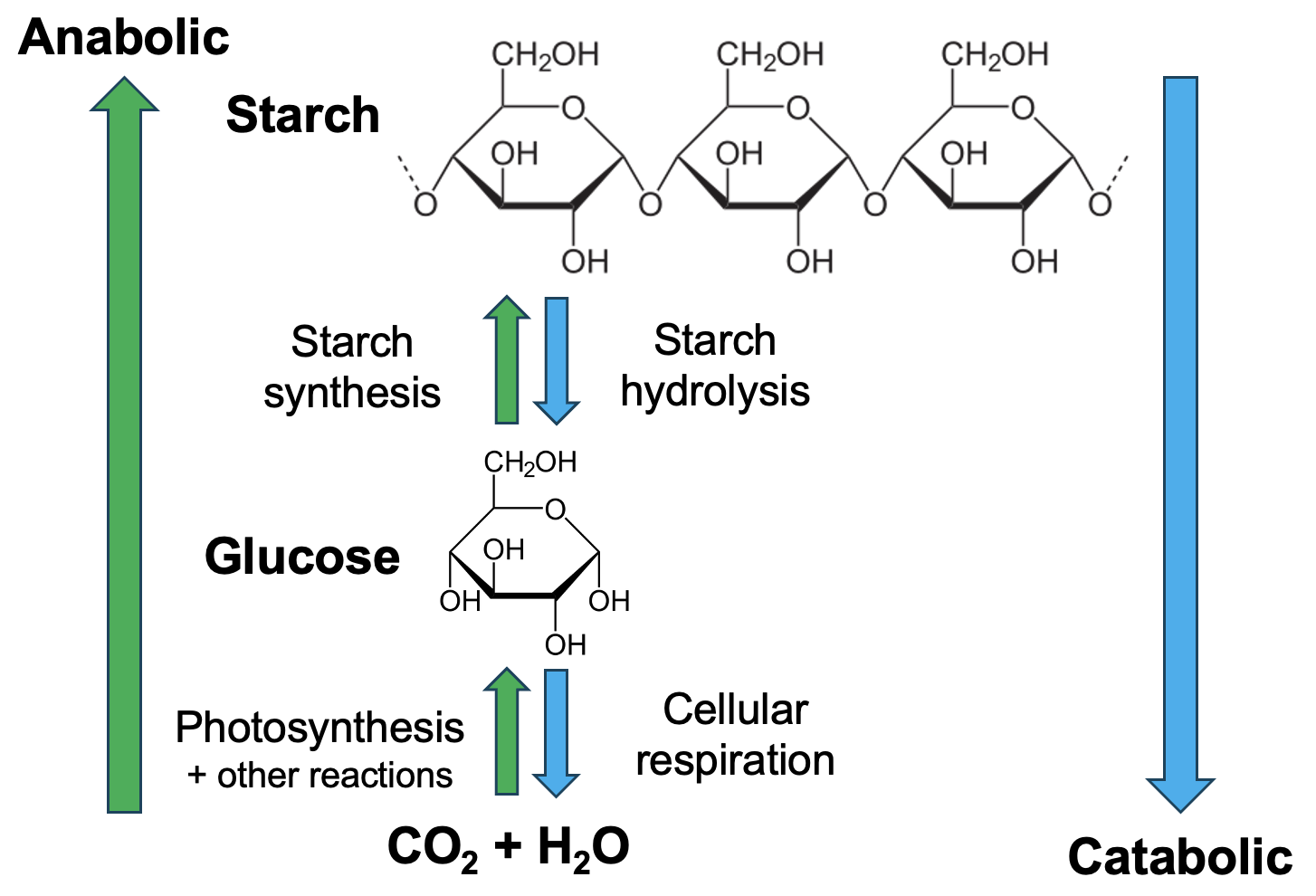
Both the pathway to produce glucose and the pathway to produce starch are anabolic pathways, in which smaller and simpler molecules are converted to larger, more complex molecules. Applying our energetics knowledge from Chapter 10, we know that these synthesis pathways contain many energetically unfavorable reactions (with positive ΔGs) that will require energy input to occur.
On the right side of Figure 12.2, a different set of reactions are occurring in a different direction. Catabolic pathways are breakdown pathways, using chemical reactions to convert larger, more complex molecules into smaller and simpler outputs. For example, starch can be broken down into glucose monomers via hydrolysis reactions. Next, the reactions of cellular respiration break down glucose into CO2 and water. In contrast to anabolic pathways, catabolic pathways reactions typically release energy.
Recall a key principle from Chapter 10—energetically unfavorable reactions, such as those in anabolic pathways, will not occur on their own. However, reaction coupling, in which the unfavorable reaction is combined with an energetically favorable reaction, will be able to form the desired products if this coupling makes the overall ΔG negative. Just like active transport proteins are essential for coupling favorable processes with unfavorable molecule movements, reaction coupling requires enzymes to link together the coupled reactions. Another principle to be aware of is that catabolic pathway reactions will release energy into the environment, not performing any useful work, unless they are coupled to reactions that can absorb some of the free energy.
Section 12.2 Coupled Reactions with ATP and NAD(P)H
For both anabolic and catabolic pathway reactions, reaction coupling is how energy is captured from reactions that release energy and transferred to reactions that require energy. Fortunately, despite the complexity of metabolism overall, reactions are often coupled to the same types of molecules—ATP and NADH (or the similar molecule NADPH). ATP and NADH are also called energy management molecules, as they are molecules that temporarily store energy so it can be used in other chemical reactions.
Before going into more details about these molecules and their reactions, we first need to explain how metabolic reactions are commonly depicted. Figure 12.3 shows a generic reaction in which a substrate reacts with ATP to produce a phosphorylated substrate and ADP. The standard way of writing out this reaction is shown in Figure 12.3a.
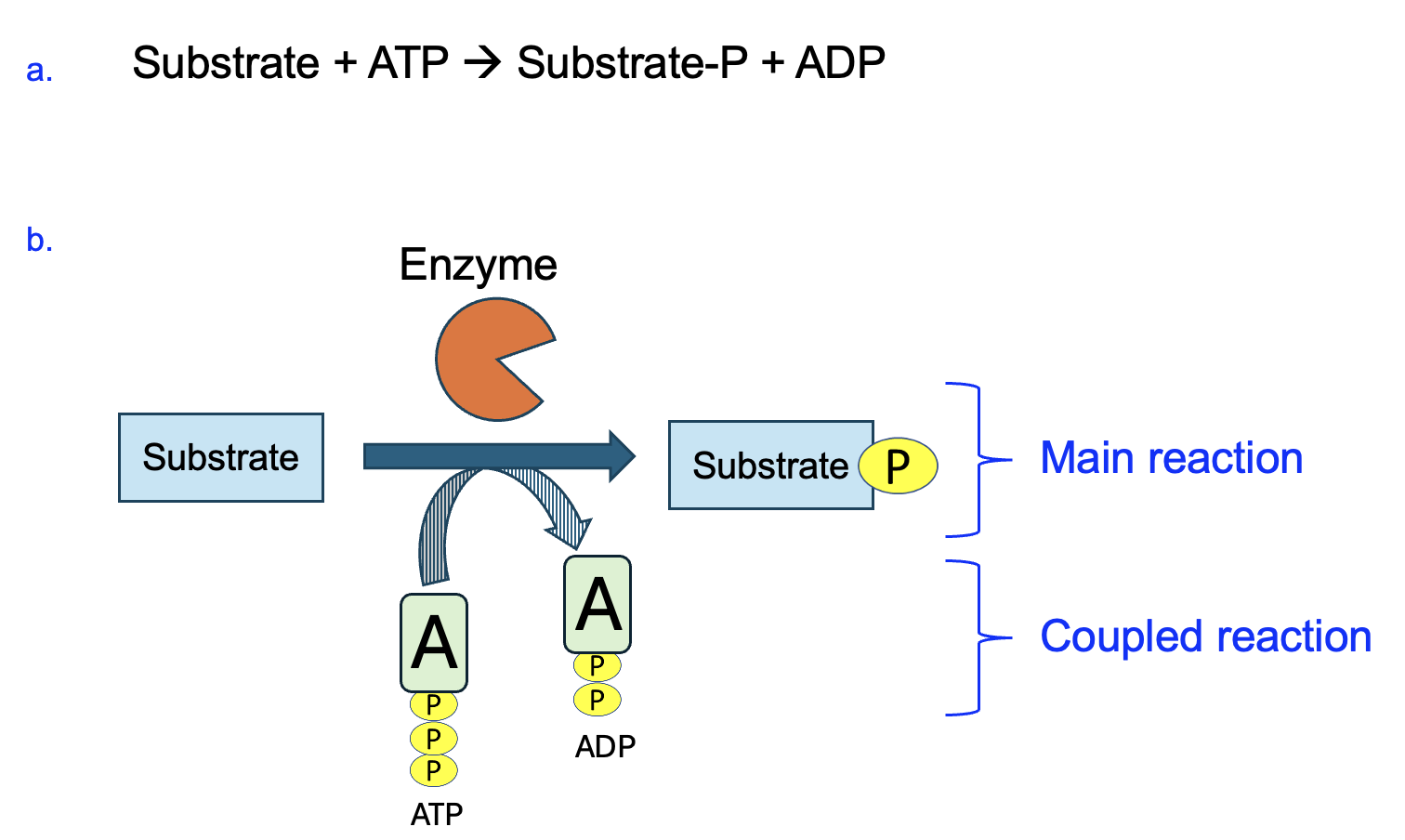
In metabolism, given that so many reactions are coupled to ATP the convention is to show the ATP reaction (the coupled reaction) on a curved arrow, as shown in Figure 12.3b. This allows for more focus on the variable and therefore more interesting main reaction (straight arrow reaction in Figure 12.3b). This representation takes some getting used to, but just remember that everything on the flat side of the straight and curved arrows is a reactant, and everything on the pointed side of each arrow is a product.
Figure 12.4 shows the reaction involving ATP that we also encountered in Chapter 10. ATP, adenosine triphosphate is dephosphorylated (loses a phosphate) to become ADP (adenosine diphosphate) and Pi (inorganic phosphate).
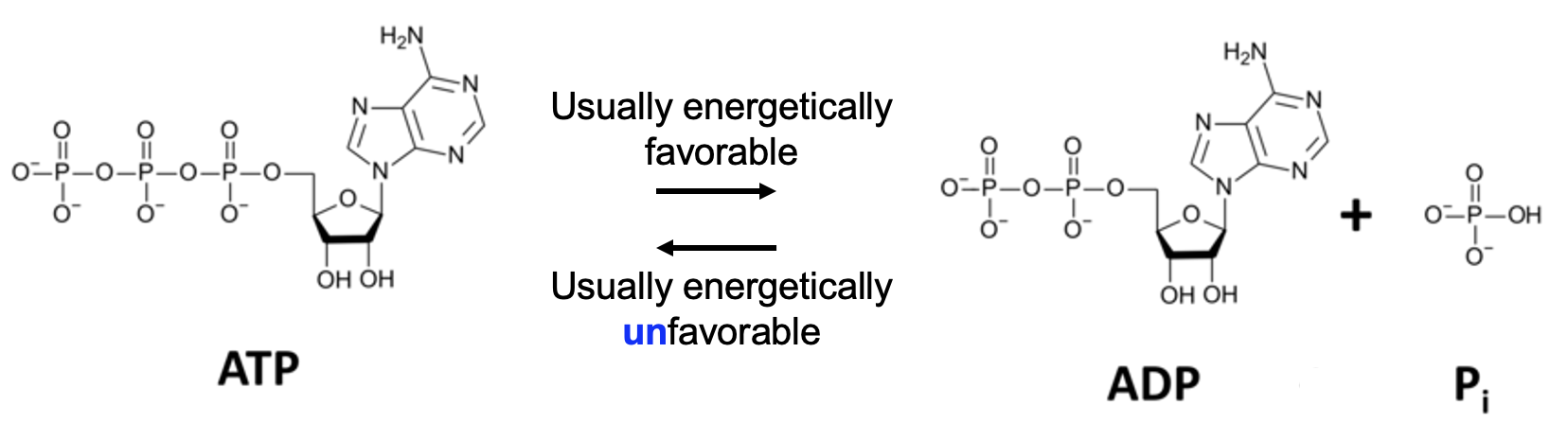
In a metabolic pathway reaction, that phosphate is usually attached to another molecule as shown in Figure 12.3. Under standard conditions, the forward reaction ATP –> ADP is typically energetically favorable, so it can provide energy for reactions that require energy input. This reaction also works in the reverse direction, with the phosphate being added to ADP to produce ATP. However, the reaction is this direction requires energy input and is therefore energetically unfavorable.
The reaction involving NADH or NADPH is very different, as it involves the transfer of electrons (and in this case, a proton as well) instead of phosphates. Look carefully at the ring structure at the top of each molecule in Figure 12.5a. NAD+ has a positive charge on the nitrogen of the ring, while NADH has a no charge and one additional H.
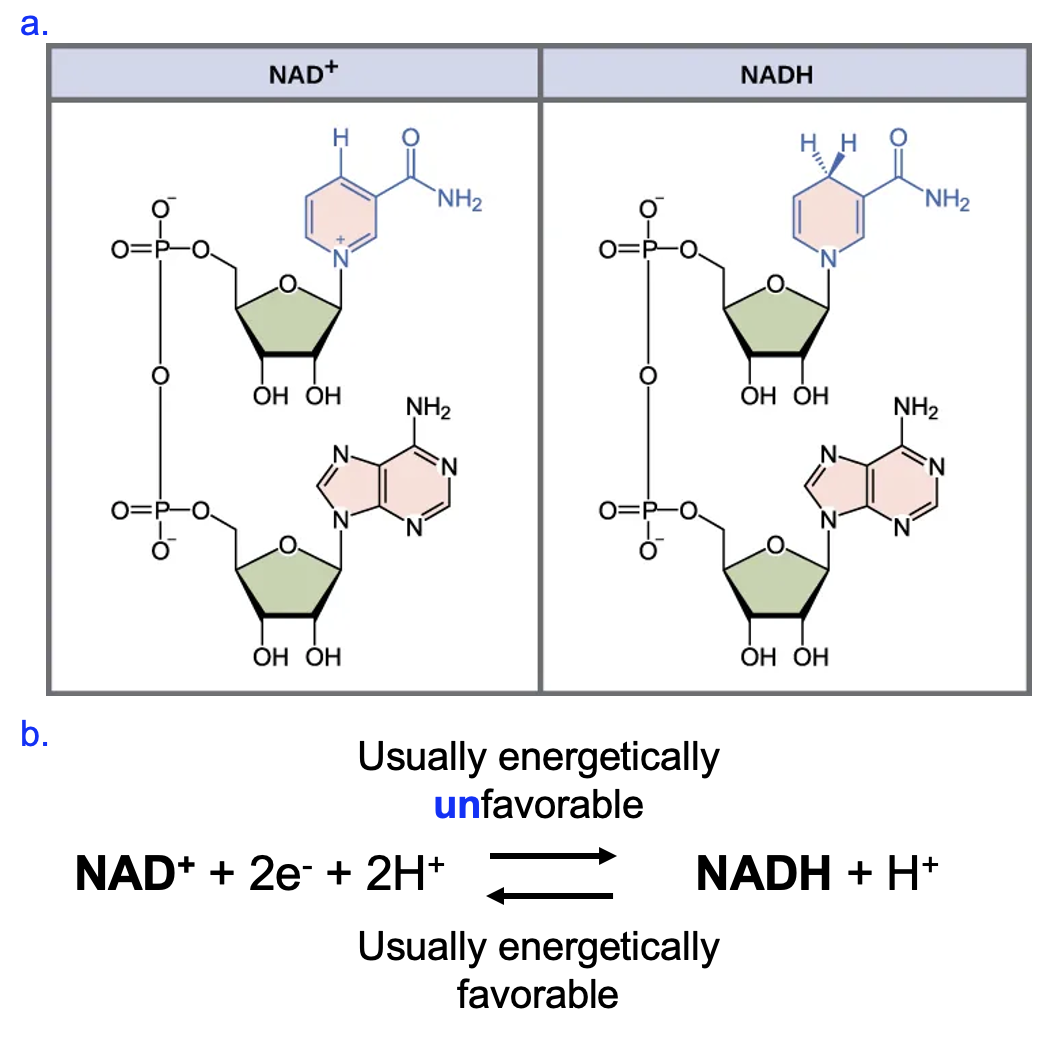
In the reaction to produce NADH (Figure 12.5b), two electrons and two protons are removed from another substrate (not shown here), and the two electrons and one proton are added to NAD+ to neutralize the charge and provide the hydrogen that was added (remember that a hydrogen atom is one electron and one proton). The remaining proton is another product. The reaction involving NADPH is identical, the only difference is that NADPH has a phosphate group attached to the 2’ carbon of the ribose sugar at the bottom of the molecule. As a result, we may write NAD(P)H to indicate the general reaction occurring to both NADH and NADPH.
Another name for reactions that involve electron transfers is reduction-oxidation (redox) reactions. When a molecule loses electrons, it is said to be oxidized. Meanwhile, the molecule that gains those electrons is said to be reduced. These names seem counterintuitive in their meaning, and a good way to keep this clear in your mind is to use the mnemonic OILRIG, which stands for Oxidation Is Loss, Reduction Is Gain. Going back to the reaction in Figure 12.5b, we can see that NAD+ gains electrons to from NADH, so we could say that NAD+ is being reduced to NADH. In the reverse reaction, NADH is being oxidized to NAD+, as it loses electrons in the process. Under typical cellular conditions, the oxidation of NADH to NAD+ is generally energetically favorable, while the reduction of NAD+ to NADH is generally energetically unfavorable.
Section 12.3 Reactions Coupled to Anabolic and Catabolic Pathways
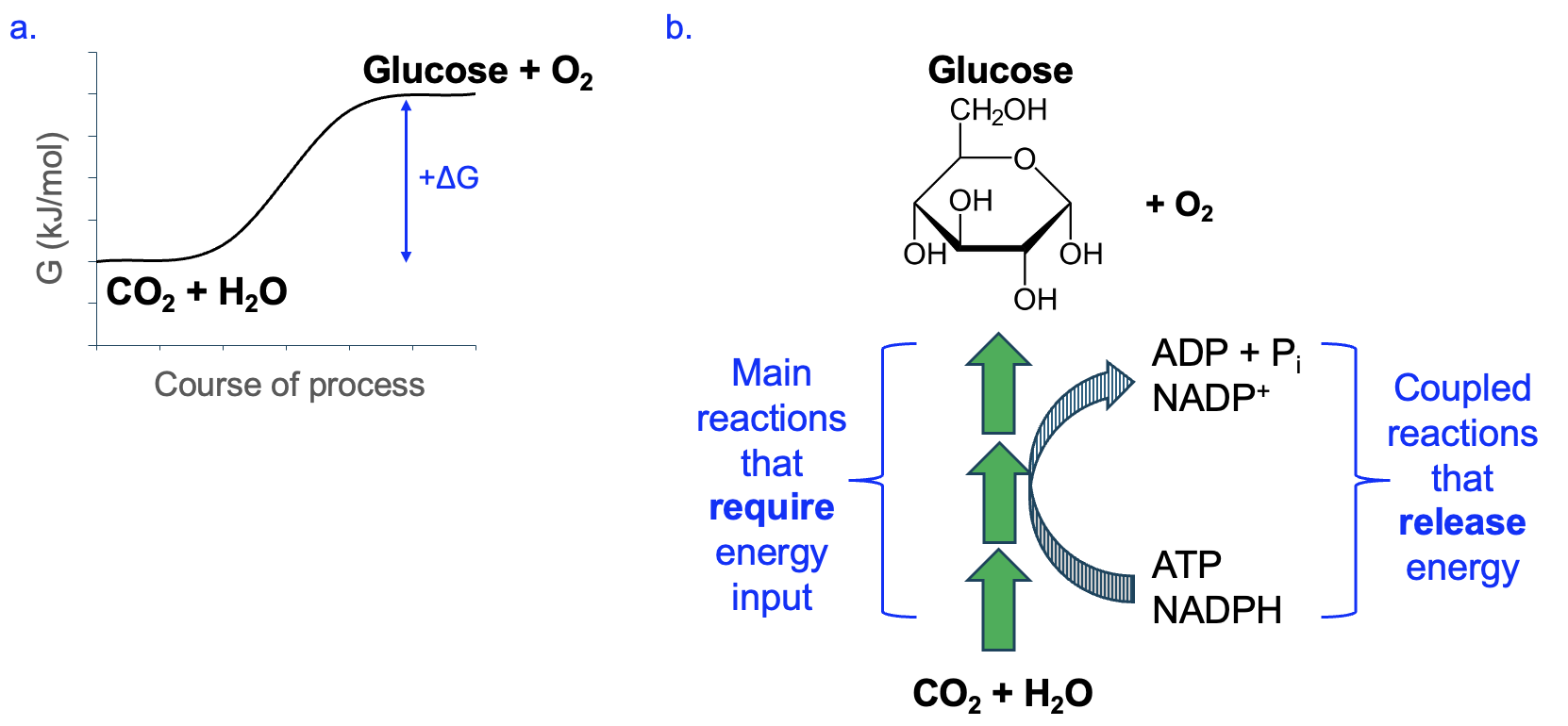
Let’s return to the anabolic and catabolic pathways shown in Figure 12.2 with this new information. We already knew that anabolic pathways require energy input, which we could also depict using a graph of the uncoupled free energy change (Figure 12.6a). Moreover, we know that for these reactions to occur and form products, these will need to be coupled with energetically favorable reactions. Based on what we know about ATP and NADPH, their reactions are energetically favorable in the direction of ATP dephosphorylation (ATP [latex]\rightarrow[/latex] ADP) and NADPH oxidation (NADPH [latex]\rightarrow[/latex]NADP+). Figure 12.6b shows the typical energetic coupling that occurs with anabolic pathways, allowing energy released by the coupled reactions to be used for the synthesis reactions that need it. While it is true that ATP is broken down to ADP and Pi in these reactions, the overall multi-step pathway to which reaction like these are coupled is anabolic, creating monomers and macromolecules for the cell.
Conversely, a catabolic pathway is overall energetically favorable (Figure 12.7a), and some of the energy released can be captured in coupled reactions. The phosphorylation of ADP to produce ATP and the reduction of NAD+ to NADH are energetically unfavorable on their own, but can be made favorable through energetic coupling with catabolic pathway reactions (Figure 12.7b).
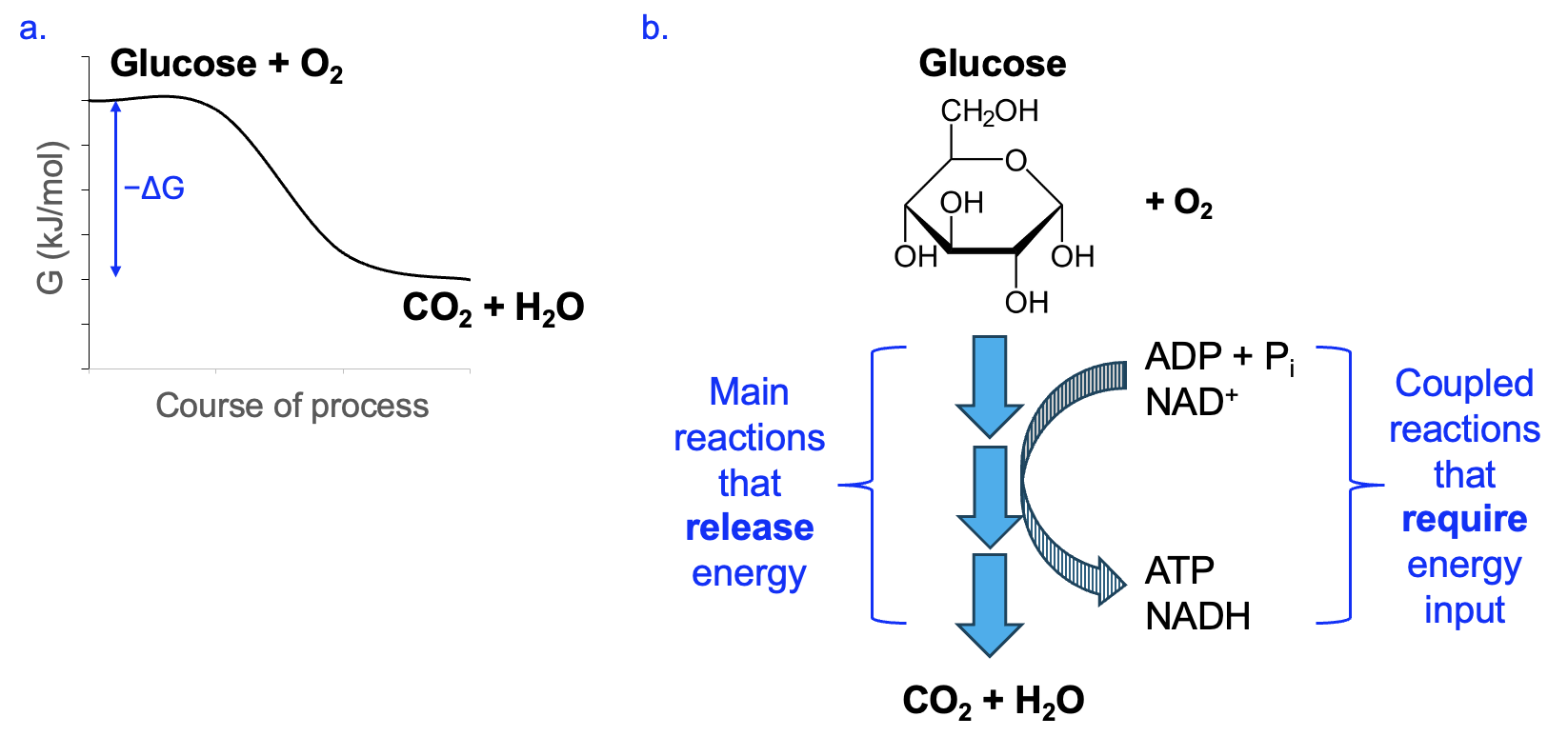
We will explore some specific reactions in a later section of this and later chapters, and you will see that ATP and NAD(P)H figure prominently in many of these. You can use the known energetics of their reactions to predict the energetics of the reactions they are coupled with. For example, if a pathway is associated with net ATP and NADH production, that is a good indicator that you are looking at a catabolic pathway, since energy was provided by the main reactions to make ATP and NADH.
Model of reaction coupling in anabolic and catabolic pathways
ACTIVITY IS UNDER CONSTRUCTION
Drag the terms to the correct location on the image.
Section 12.4 Free Energy Changes in Coupled Reactions
Once you are familiar with the energetics of coupled reactions, you can use this to make predictions about other aspects of the reaction. One key element is the free energy change, or ΔG, occurring due in the reaction. A given reaction can either have a negative ΔG, meaning the products of the reaction have lower energy than the reactants, or a reaction can have a positive ΔG, meaning the products have higher free energy than the reactants. If we observe a coupled reaction, we can assume that one part of the reaction is energetically unfavorable on its own, and therefore would not occur without coupling. Since we know the energetics of some common coupled reactions, we can make predictions about the energetics of the rest of the reaction.
Reactions Involving ATP
Let’s practice with some individual reactions from metabolic pathways. Figure 12.8a shows a reaction that should be familiar with you—the phosphorylation of glucose using ATP. Writing this in our coupled reaction format helps show the energetics at work (Figure 12.8b). On the curved arrow, ATP is dephosphorylated to ADP, which we know to be energetically favorable. Because this is a coupled reaction, we can then infer that the reaction on the straight arrow, glucose gaining a phosphate, is the energetically unfavorable reaction that requires energy input. Since this reaction has a positive ΔG, the glucose reactant is lower in free energy than the phosphorylated glucose product, as shown in Figure 12.8c.
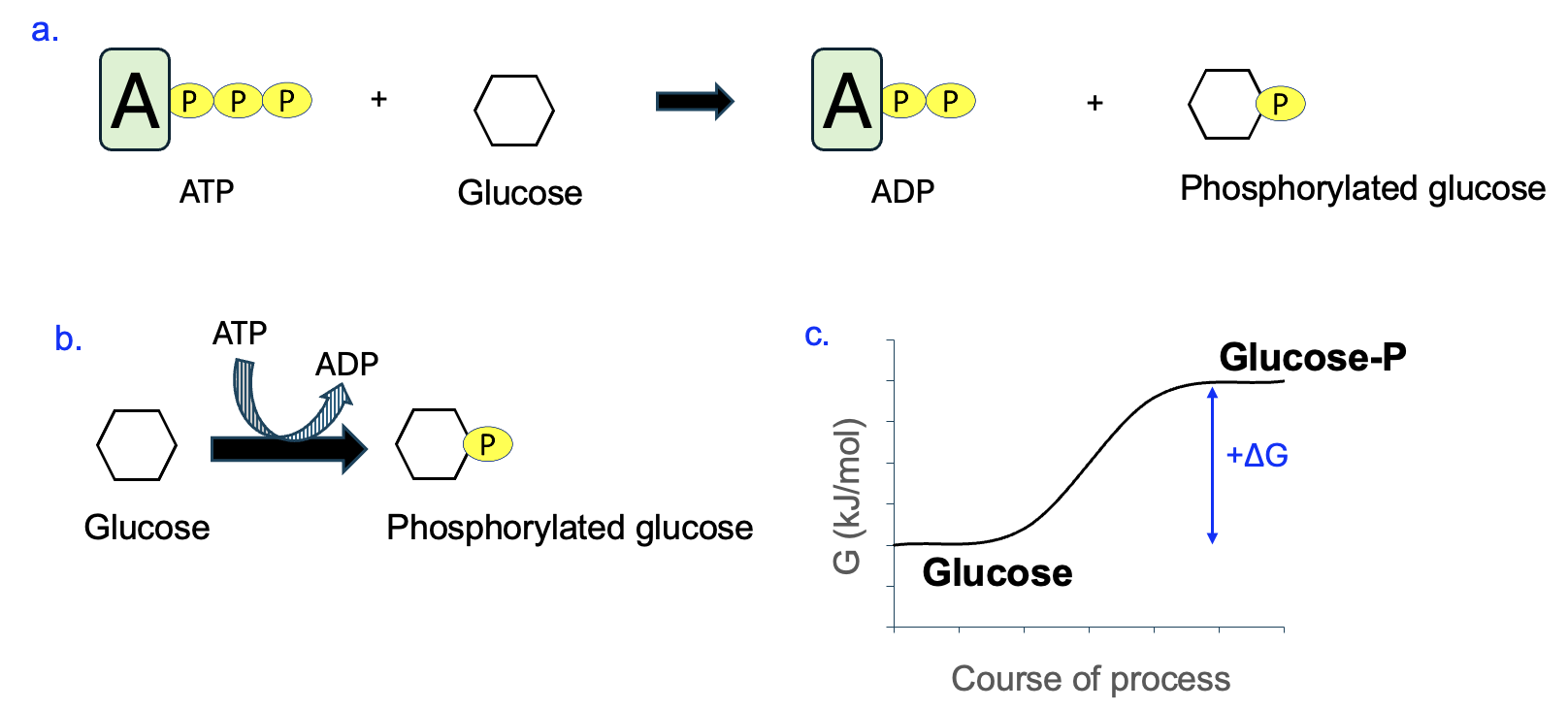
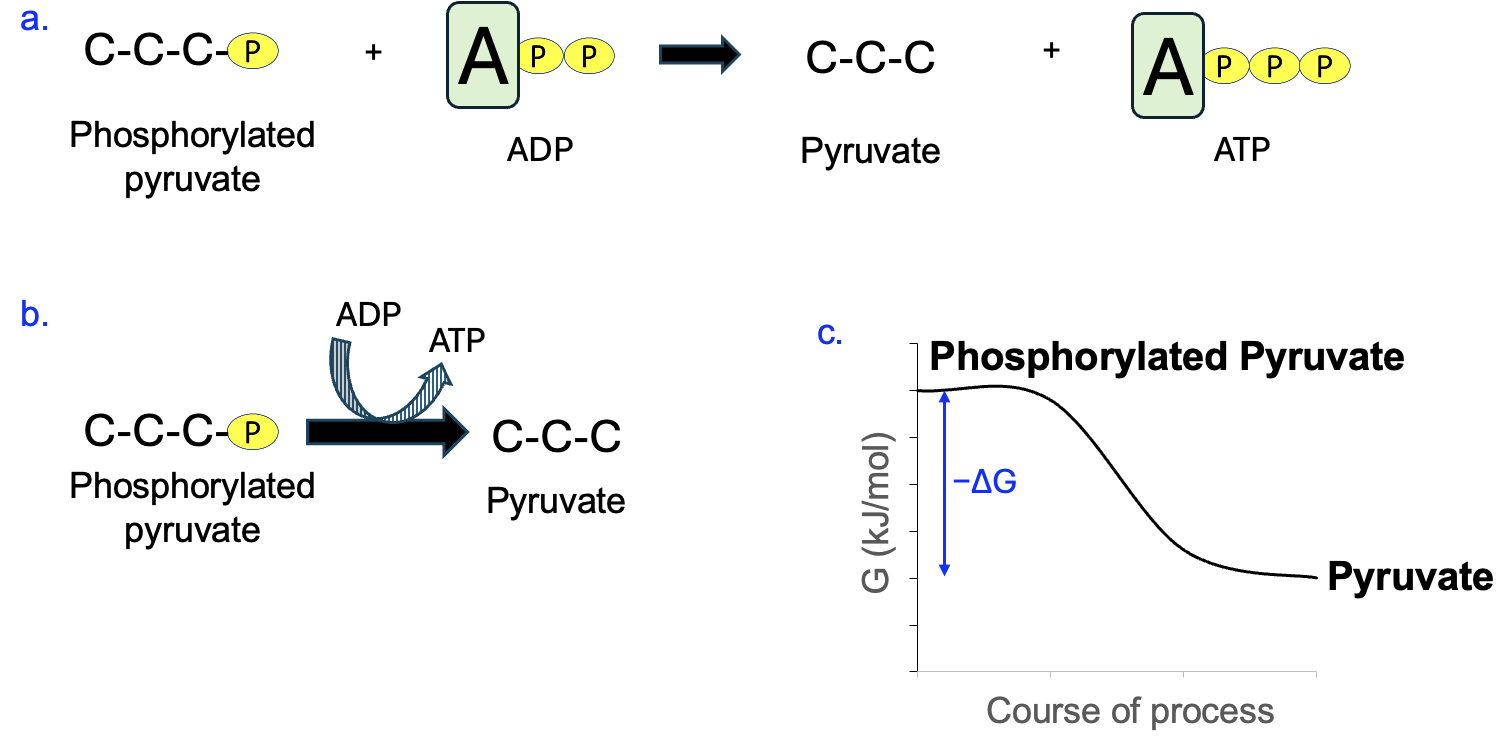
Figure 12.9a shows another reaction involving ATP, except some reactants and products are reversed. The detailed chemical structures are not shown, but we can see that a small organic molecule with a phosphate attached reactions with ADP to produce ATP and the small organic molecule without the phosphate. Here, the coupled reaction is ADP [latex]\rightarrow[/latex] ATP (Figure 12.9b), which we know to be energetically unfavorable. Therefore, the energy input for this reaction must come from the main reaction with a negative ΔG. Thus, phosphorylated pyruvate has higher free energy than the pyruvate, and energy that’s released from that reaction is what is used to produce ATP.
Reactions Involving NAD(P)H
Now let’s look at some metabolic reactions involving NAD(P)H. We have already observed that NADH can be oxidized to NAD+, and NAD+ can be reduced to NADH. In later figures, we will look at the full reactions to see what is being reduced when NADH is being oxidized, or what is being oxidized so that NAD+ can be reduced. But understanding these reactions requires more explanation of how redox reactions appear in metabolism, and amending the definitions in the mnemonic OILRIG.
Figure 12.10 shows several organic molecules arranged from most reduced (methane) to most oxidized (CO2). We defined OILRIG as Oxidation Is Loss, Reduction Is Gain, with loss and gain relating to electrons transferred in the reaction. However, comparing each of the structures in Figure 12.10, no electrons appear to be fully lost or gained, as each carbon has four total covalent bonds and all atoms are neutral with no full positive or negative charges.
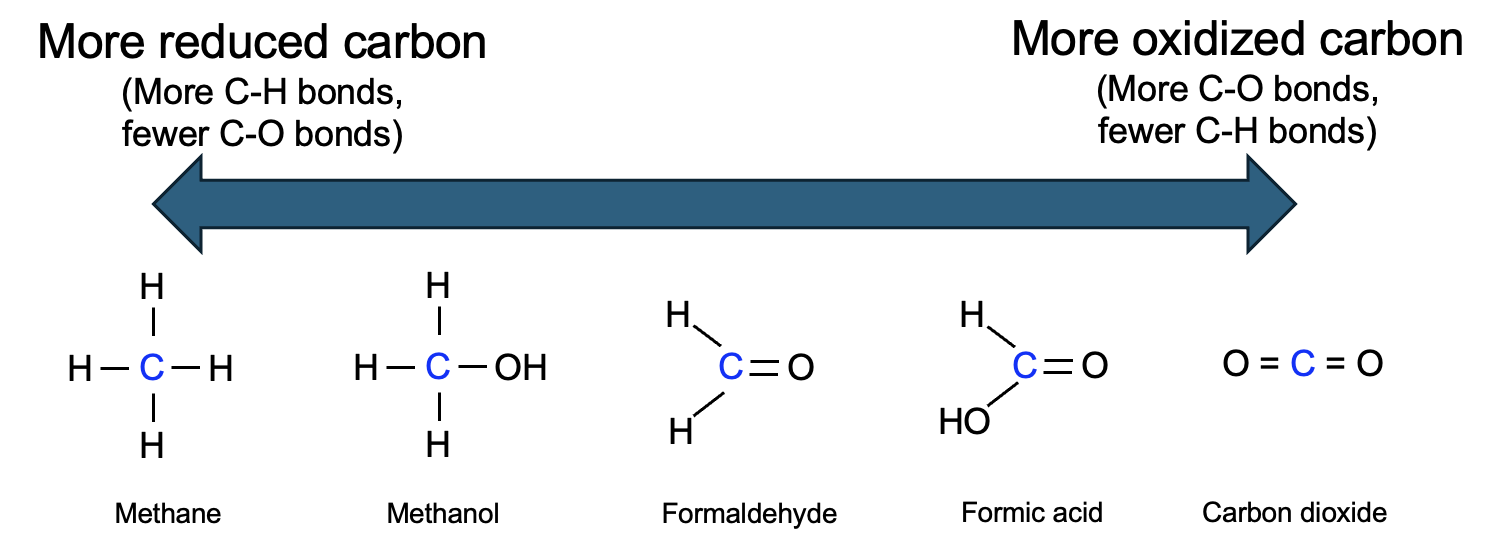
Focus carefully on the carbon in each structure, and keep in mind the property of electronegativity, or an atom’s affinity for electrons, from Chapter 2. Carbon and hydrogen have similar electronegativities, so they equally share electrons in a covalent bond. Conversely, oxygen is much more electronegative than carbon, so in a covalent bond between C and O, the electrons are localized closer to oxygen than carbon. In CO2, all of carbon’s bonds are to oxygen, and thus carbon in this molecule has much less control over its electrons. This makes the carbon in CO2 partly oxidized. Meanwhile, the carbon is methane is more reduced, given that it is sharing electrons equally with H.
We can amend our interpretation of OILRIG to capture this idea—Oxidation Is Loss of electrons or loss of bonds between carbon and hydrogen, Reduction Is Gain of electrons or gain bonds between carbon and hydrogen. Notably, when carbon is oxidized by losing a bond to hydrogen, the bond is sometimes replaced with a new bond to oxygen. So oxidation can also be observed as a gain of bonds between carbon and oxygen, while reduction is the loss of those bonds (replaced with bonds between carbon and hydrogen).
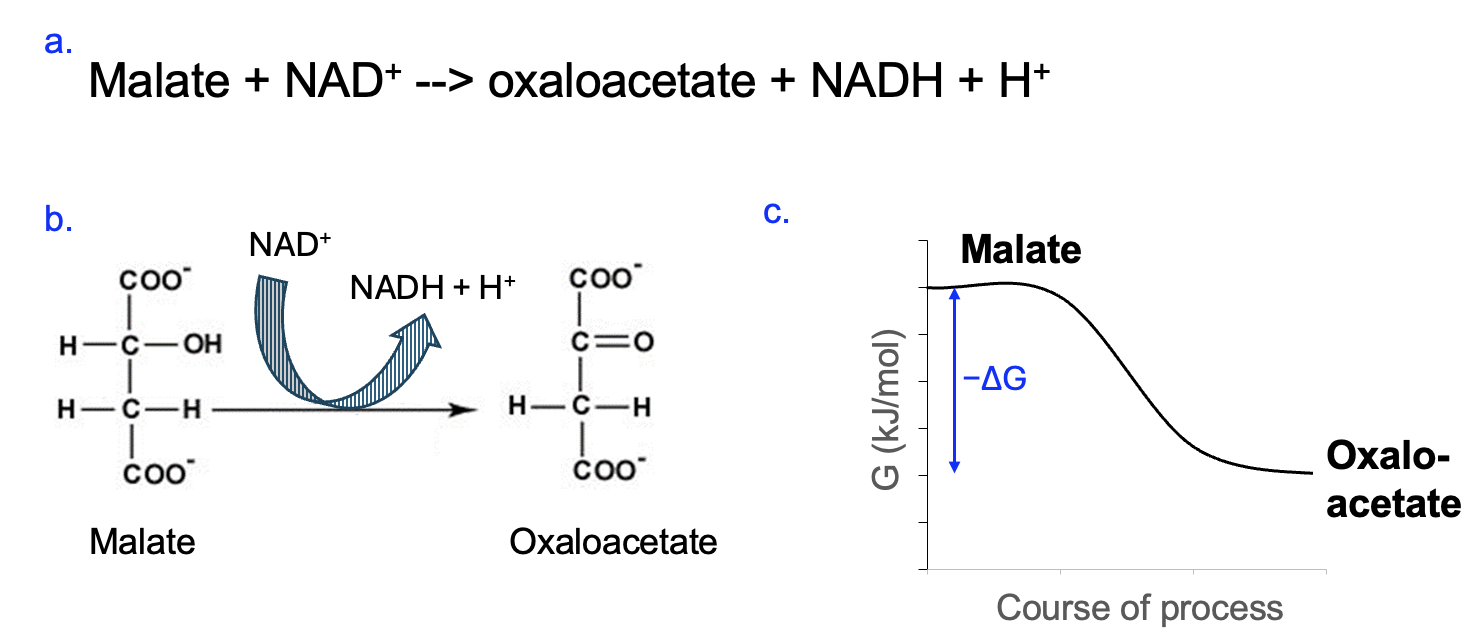
Figure 12.11 shows a reaction involving NADH in the typical reaction format (12.11a) and as a coupled reaction (12.11b). In the coupled reaction NAD+ is being reduced to NADH (gained electrons), and those electrons came from the other reactant, malate. In the main reaction, malate is being oxidized to oxaloacetate. We know this because the 2nd carbon from the top of the malate molecule loses two hydrogens, forming two bonds to oxygen in their place.
Even without the benefit of the chemical structures, if we know that NAD+ is reduced to NADH, the electrons to reduce NAD+ must come from the other reaction. Thus, if one part of a reaction is a reduction, the other part of the reaction must be an oxidation, as reduction and oxidation go together.
We can also predict the energetics of the reaction in Figure 12.11. Since NAD+ [latex]\rightarrow[/latex] NADH is energetically unfavorable, the energy input must come from the conversion of malate to oxaloacetate. The free energy graph, therefore, corresponds to a reaction with a negative ΔG. One could also predict, given the NAD+ [latex]\rightarrow[/latex] NADH reaction, that this reaction is part of a catabolic pathway. Indeed, this is a reaction from the Citric Acid Cycle, which is part of cellular respiration, a major catabolic pathway in cells (Figure 12.2).
Figure 12.12 shows a more complicated reaction involving NADPH. You may recognize very little in the main reaction, but you also don’t need much information about these molecules to make the same types of predictions we have made previously.
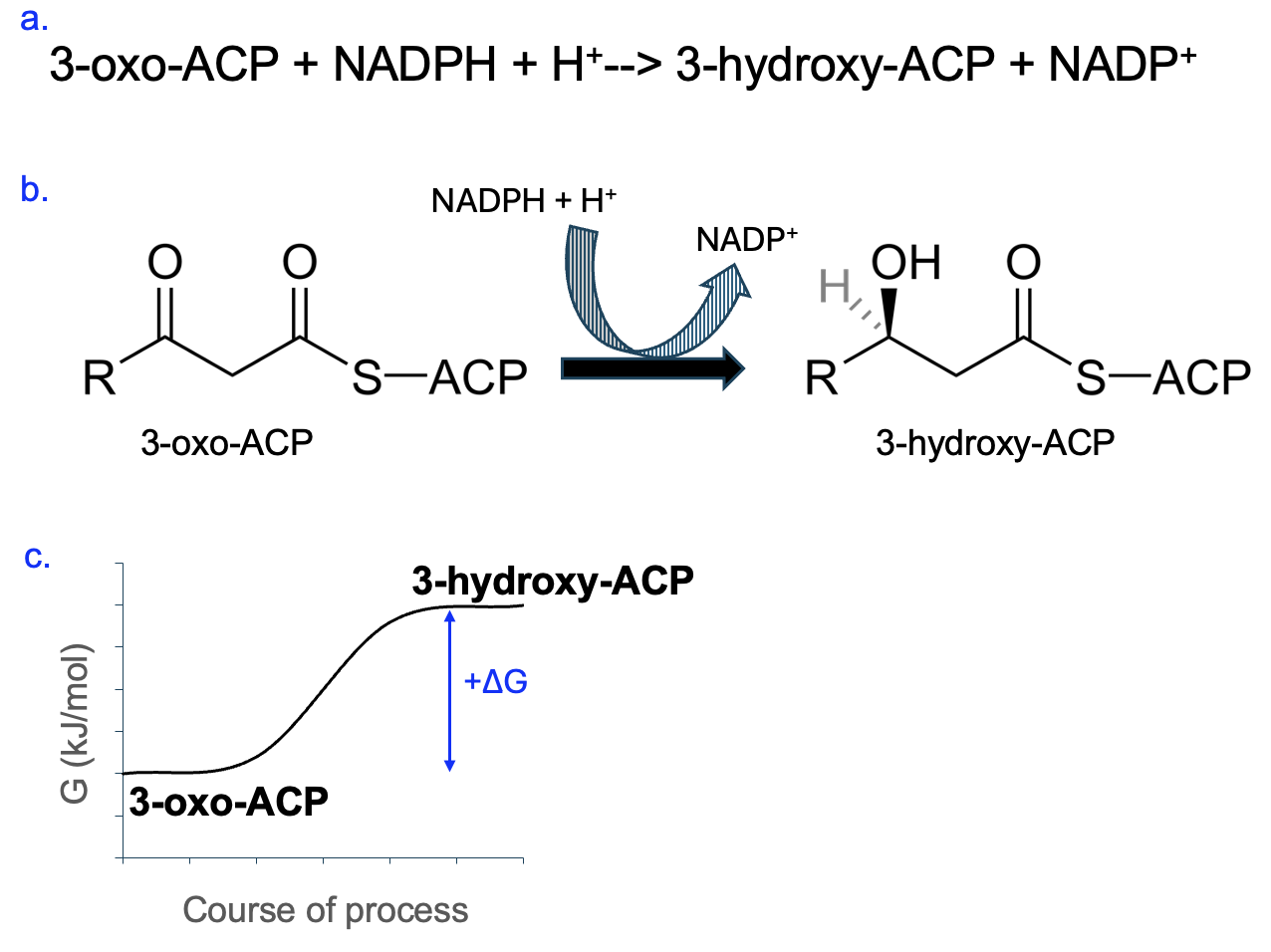
Focus first on the coupled reaction, NADPH [latex]\rightarrow[/latex] NADP+. This is an energetically favorable reaction with a negative ΔG, so the other reaction must have a positive ΔG. Further, NADPH [latex]\rightarrow[/latex] NADP+ is an oxidation, so the other reaction must be a reduction, and we should look for hydrogens being added to a carbon as the reactant is converted to product. Can you spot the reduction that occurs?
Section 12.5 Metabolic Pathways and Energetic Favorability
The examples in section 12.3 are based on standard conditions, which assume certain concentrations of reactants and products. However, in a cell, (1) these reactions occur as part of larger metabolic pathways, and (2) conditions often vary from standard conditions. Both of these impact the energetic favorability (ΔG) of reactions. As we consider these impacts, keep in mind a very fundamental rule: for a reaction to occur, there must be sufficient reactants. This can be explained using Le Chatelier’s principle: just as adding reactants can make product formation more favorable, removing reactants will make the formation of products less favorable. A reaction that is very energetically favorable under standard conditions can be made very unfavorable and will not form products if reactant levels are too low.
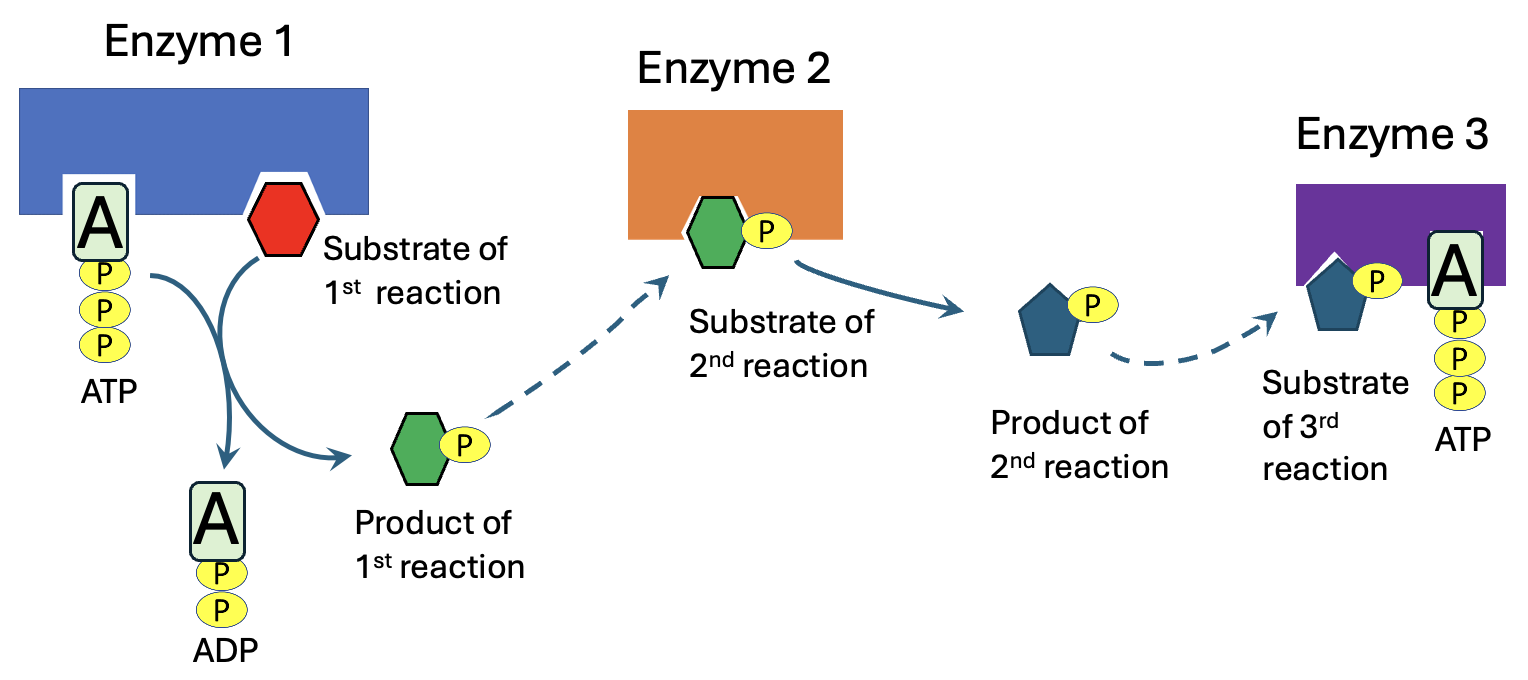
Figure 12.13 shows a series of three reactions that are in a metabolic pathway. Each reaction is catalyzed by a different enzyme, and the product of the first reaction is the substrate for the 2nd enzyme, which in turn produces a product that is the substrate for the 3rd enzyme. What are the advantages of having reactions linked in this way?
Let’s first consider a simple reaction, where A and B react to form C and D in a reversible reaction. In Chapter 10, we learned that that the ΔG indicates how far a system is from equilibrium and in what direction. If a chemical reaction has a negative ΔG, then to reach equilibrium, reactants will be converted to products, and this might happen more rapidly if an enzyme is present. In this case, A and B collide with each other and are converted to C and D. Over time, then, what happens to the concentrations of the reactants and products? Well, A and B will decrease in concentration as C and D increase in concentration. As a result, there are fewer reactants to react with each other. Additionally, the increase in product concentrations makes it more likely that those products will collide with each other to form reactants by the reverse reaction. If an enzyme is present, it will catalyze the reaction in both directions, but net reactions will proceed in the direction of the negative ΔG.
Thus, while it might seem counterintuitive, the above example indicates that as the forward reaction occurs, the forward reaction becomes less favorable due to decreased reactants, while the reverse reaction becomes more favorable due to increased products. Eventually, the system reaches equilibrium, where the forward reaction happens at the same rate as the reverse reaction and ΔG=0. Remember, equilibrium is not when concentrations of A and B are equal to C and D. Rather, it when concentrations of reactants and products are at concentrations relative to each other where no net changes are occurring, which we will call equilibrium concentrations.
If a cell needs a constant supply of the products from a specific reaction, that means the reaction needs to be favorable in the forward direction. In the example above, this could be accomplished by maintaining concentrations of reactants, A and B, that are above their equilibrium concentrations, increasing the likelihood of these molecules colliding and reacting to form products. Additionally, cells need to maintain concentrations of products C and D that are lower than equilibrium concentrations, so they are less likely to react with each other and form A and B. This could be accomplished by converting C or D to something else via another chemical reaction. Another option to decrease the concentrations of products is through membrane transport—cells that move products into a specific compartment of the cell or export the products.
Going back to Figure 12.13, as enzyme 1 acts, product 1 is made. Over time, if no other reactions occur, product 1 accumulates. As a result, the reaction to produce product 1 becomes progressively less favorable, eventually reaching equilibrium. In the pathway shown in Figure 12.13, though, there is a second enzyme that can use product 1, and convert it to product 2, thus keeping concentrations of product 1 low. If cells have a steady supply of reactants, the linked reactions of a metabolic pathway can keep product concentrations low, so the reactions remain favorable.
Additionally, a highly favorable reaction in a metabolic pathway can drive another reaction in the same pathway that is unfavorable on its own. Since the reactions are linked, the ΔG of a metabolic pathway is the sum of the ΔG values for each individual reaction. Let’s imagine that in the pathway shown in Figure 12.13, the ΔG values are -3 kJ/mol for reaction 1, +1 kJ/mol for reaction 2, and -2 kJ/mol for reaction 3. Reaction 2 on its own would not occur in the forward direction, given its positive ΔG, but the favorability of reactions 1 and 3 drive the reaction to occur, as the pathway has an overall ΔG of -4 kJ/mol.
Let’s apply this thinking to one metabolic pathway from the start of this chapter, cellular respiration. The inputs are sugars and O2 and the products are CO2 and water. We will explore more details of this complex and essential process in Chapter 13, but you can already apply your knowledge and personal insights to understanding this pathway. Firstly, as mentioned previously, this reaction is highly energetically favorable under standard conditions, with ΔG = -2800 kJ/mol. Nevertheless, this reaction can be made unfavorable in the absence of reactants. How do you keep this set of reactions favorable and occurring at a reasonable rate? Well, ideally you had food at some point today, and you are undoubtedly breathing regularly to supply O2 to your cells and release the CO2 product!

