7 Model of Protein Engineering
Learning Objectives
- Predict amino acids that could interact favorably to create a protein’s tertiary structure
- Predict amino acids in a binding site that could interact favorably with a specific substrate molecule
- Use a model to explain how a change in the tertiary structure of a protein could lead to a different function
Figure 7.1 shows a model of a protein whose function is to bind to a small molecule that fits into the cavity (the small molecule is not shown). Without this specific cavity, the protein could not carry out the function of binding to this small molecule.

If one amino acid were changed in this protein, how do you think this would affect the protein’s function? Which choice below best describes your prediction? How confident are you in your prediction?
- No impact on function
- A minor change in function
- A major change in function
- All options are possible, and more information is needed
One principle to keep in mind is the broad theme of this unit—structure is connected to function. To predict the effect of the amino acid change on function, we need to know how the structure of the protein will be affected, and that is influenced by the specific amino acid change. We can use the amino acid structures, and what we have learned about their properties and interactions, to build our prediction. If the property of the new amino acid is very different from the original amino acid (for example, if a nonpolar amino acid is replaced with a polar amino acid), we might assume the protein’s structure would be different and affect the protein’s function.
We also would benefit from knowing where the change occurred in the protein in relation to our model. The model of the protein communicates which parts of the amino acid sequence are important for the function of the protein. We might predict a stronger effect on function if the change is in the cavity where the small molecule binds, as opposed to a change that is far away from the cavity and doesn’t change its shape.
However, without knowing the original or the new amino acid, or where in the protein this amino acid resides, we can’t make a strong prediction about the effect on protein structure or function. So, choice 4 is the best answer that we can confidently predict—we need more information!
Scientists use sophisticated computer modeling to make predictions about how changing an amino acid will change the structure and function of a protein. However, even with the best computer models, a prediction is just that—a prediction. To create a strong, scientifically acceptable argument about the effect of changing particular amino acid on the protein, we need to do an experiment to generate evidence of what happens to the protein’s structure and function.
Lastly—how can we change the structure of a protein? Remember that the amino acid sequence is encoded by the mRNA. The mRNA is encoded by the DNA. Therefore, to change the amino acid sequence, we must first change the DNA.
Chapter Outline
Section 7.1 Test your knowledge
Section 7.3 Protein Engineering
Section 7.1 Test your knowledge
Test your knowledge
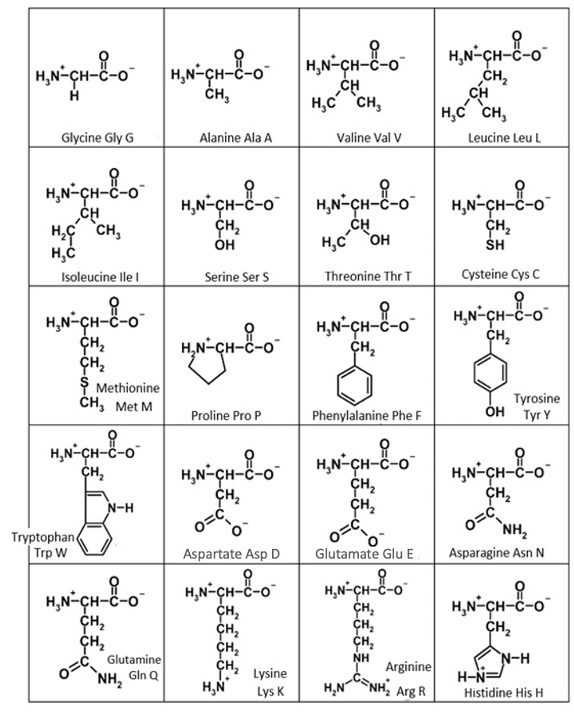
To help practice and synthesize information from this unit, consider the amino acid structures in Figure 7.2. These are organized by size rather than by chemical properties as we have seen them previously. For each amino acid, identify its R group and the key functional group in each R group. Use the property of the functional group (nonpolar, polar uncharged, positively charged, or negatively charged) to predict the strongest interaction that functional group could have with another functional group in a protein. Check your work by filling in the blanks below!
Remember, it is the interactions between R groups of different amino acids that are helping the protein achieve its tertiary structure and stabilize its folding that is essential for a protein’s function.
Exercises
Section 7.2 Enzymes
To help us build a molecular understanding of structure and function, let’s focus on enzymes. You may recall from chapter 3 that enzymes are proteins that catalyze, or speed up, chemical reactions. Figure 7.3a shows a chemical reaction between two reactants to form a specific product. The blue structure in Figure 7.3b shows an enzyme with two spaces, called active sites, where each of the reactants can fit (note that each reactant has its own binding site). When a chemical reaction is catalyzed by an enzyme, biologists refer to the reactants as substrates.
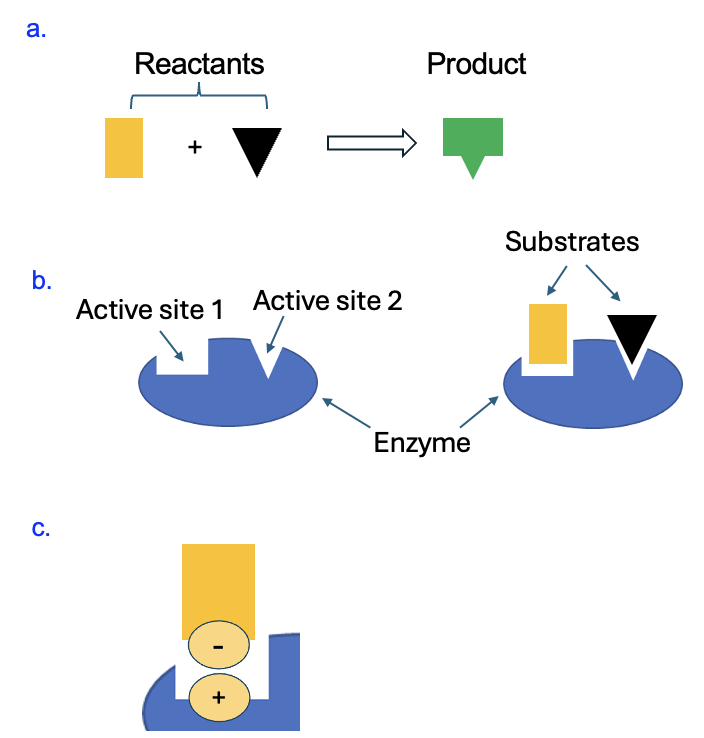
The enzyme can speed up the reaction by bringing together these substrates (more on this in a later chapter). For now, let’s focus on how the enzyme binds to these substrates. First, the enzyme must have the correct shape (tertiary structure) to bind each substrate and facilitate the reaction. The structure of the enzyme is due to its amino acid sequence (primary structure), which is in turn encoded by the DNA. Second, the properties of the amino acids that form the active site must be compatible with the substrates. If, for example, as shown in Figure 7.3c, one of the substrates has a negatively charged group, it would best interact with an enzyme that has a positively charged amino acid at the active site. Then an ionic interaction could stabilize the binding of the substrate to the enzyme so the reaction can occur.
While this is a made-up enzyme and reaction, in cells, the products of enzyme-catalyzed reactions in cells contribute to the traits, or phenotypes of cells. Back in Chapter 4, we explored two strains of the same bacteria species that nevertheless have very different phenotypes. To recap, bacteria of the R strain were rough in appearance and was nonvirulent, meaning it did not cause a lethal infection. In contrast, the S strain bacteria were smooth in appearance and were virulent. The smooth appearance of the S strain is due to the presence of a capsule that surrounds the bacteria (another phenotype!), preventing the immune system of the infected host from neutralizing the infection.
When R strain bacteria were transformed by DNA extracts from the S strain bacteria, they gained the same capsule, which is a polysaccharide. At the time, this was a confusing result—how could DNA, a nucleic acid, cause the phenotype of having a polysaccharide (a carbohydrate) capsule? We can make sense of this with the knowledge we have of gene expression and how macromolecules are formed. In the S strain DNA used to transform the R strain bacteria, there is a gene that encodes an enzyme. That enzyme creates covalent glycosidic bonds between sugar monomers to produce a polysaccharide capsule that allows the bacteria to be virulent.
Section 7.3 Protein Engineering
Now that we recapped the role of enzymes and how they achieve their structure, we can apply this information towards building enzymes with novel therapeutic potential. We will follow the example of the work carried out in the lab of Dr. Francis Arnold, who is the 2018 Nobel Laureate in chemistry. She works in the fields of chemical engineering, bioengineering, and biochemistry, among many of her roles. The focus of Dr. Arnold’s research is on an enzyme called cytochrome P450, which is part of a large family of enzymes that carry out a large variety of metabolic reactions in the body that are very important to the field of medicine. Figure 7.4a shows a ribbon model of this protein, and you can observe its tertiary structure containing several alpha helices and a few beta sheets. This model also shows a bound substrate in top center part of the protein.
Remember that while the secondary structures arise from hydrogen bonds between atoms of the peptide bond, the overall three-dimensional structure and the active site structure are due to interactions between the R groups of amino acids within the chain. Remember that at the active site, the amino acids must be complementary in properties to the substrate to allow for its binding, which would then allow the chemical reaction to be facilitated by the enzyme. It follows, therefore, that changing the shape of the enzyme, by changing key amino acids in its structure, would potentially allow the enzyme to bind to different substrates and carry out a different chemical reaction. Of course, to change the amino acid sequence, we first need to change the DNA sequence encoding the enzyme. This is the key focus of Dr. Francis’s research, as summarized in this news article: can changes to the active site of the cytochrome enzyme allow it to carry out different, and perhaps useful chemical reactions?
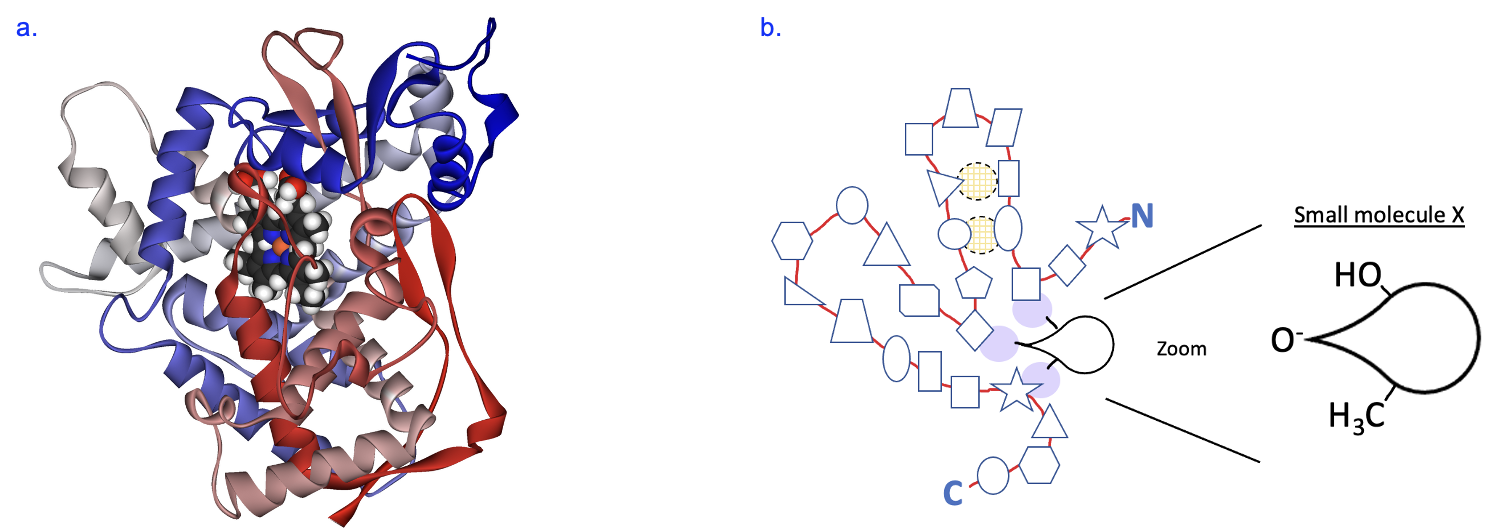
To model this in class, we will use a simpler model of the enzyme as represented in Figure 7.4b. This model represents a long chain of amino acids joined by peptide bonds, going from N terminus to C terminus. Each shape along the chain represents a different amino acid, and this protein folds into a shape that allows it to interact with a particular small molecule. We will use this model to address two questions. First, what causes this protein to fold up in the way that it does? Near the top of the enzyme, we see a group of four amino acids form intramolecular interactions (indicated by light shading with dashed black outline). In the activity, we will make some predictions about what amino acids are present at these locations and how the R groups of these amino acids interact to make this tertiary structure. Second, we will focus on the amino acids that will interact with a specific small molecule (Substrate X). Based on the chemical properties of the small molecule, we will make predictions about what R groups need to be present in the enzyme to facilitate the interaction with this substrate. Lastly, we will consider how we can change amino acids at the active site to change the small molecule that it binds to. If successful, this could result in a new reaction product and a new function for our enzyme.

